HPR1 gene mutant and application thereof for preparing diagnostic reagent for deafness
A technology of diagnostic reagents and diagnostic kits, applied in application, genetic engineering, plant genetic improvement, etc., can solve problems such as aggravation, delayed disease, and lack of HPR1.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Verification of the correlation between HPR1 and deafness diseases
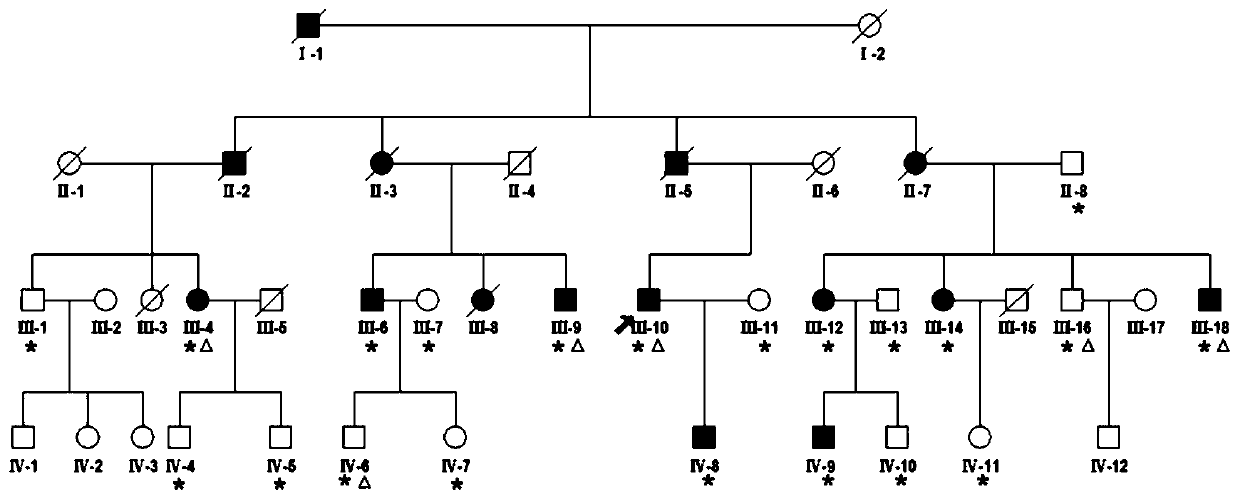
[0051] The first step, sample preparation: clinically found a family of acquired deafness (such as figure 1 shown), in which 15 patients with acquired deafness have been diagnosed, the peripheral blood samples of deaf patients (experimental group, 9 people) and non-deaf individuals (18 people) in the family were extracted respectively, and the total RNA of each sample was extracted by TRIzol method, and Store at -80°C until use.
[0052] The second step, sample exome sequencing and disease-causing mutation gene analysis: use the whole exome sequencing technology to sequence and analyze each RNA sample. The specific content includes: use the Illumina TruSeq Exome Enrichment Kit to capture the sequences of exons and surrounding intron regions of 20,794 expressed genes contained in each sample, and measure the sequences of microRNA sequences and other non-coding genes. The measuring instrument ...
Embodiment 2
[0055] Embodiment 2 prepares kit of the present invention
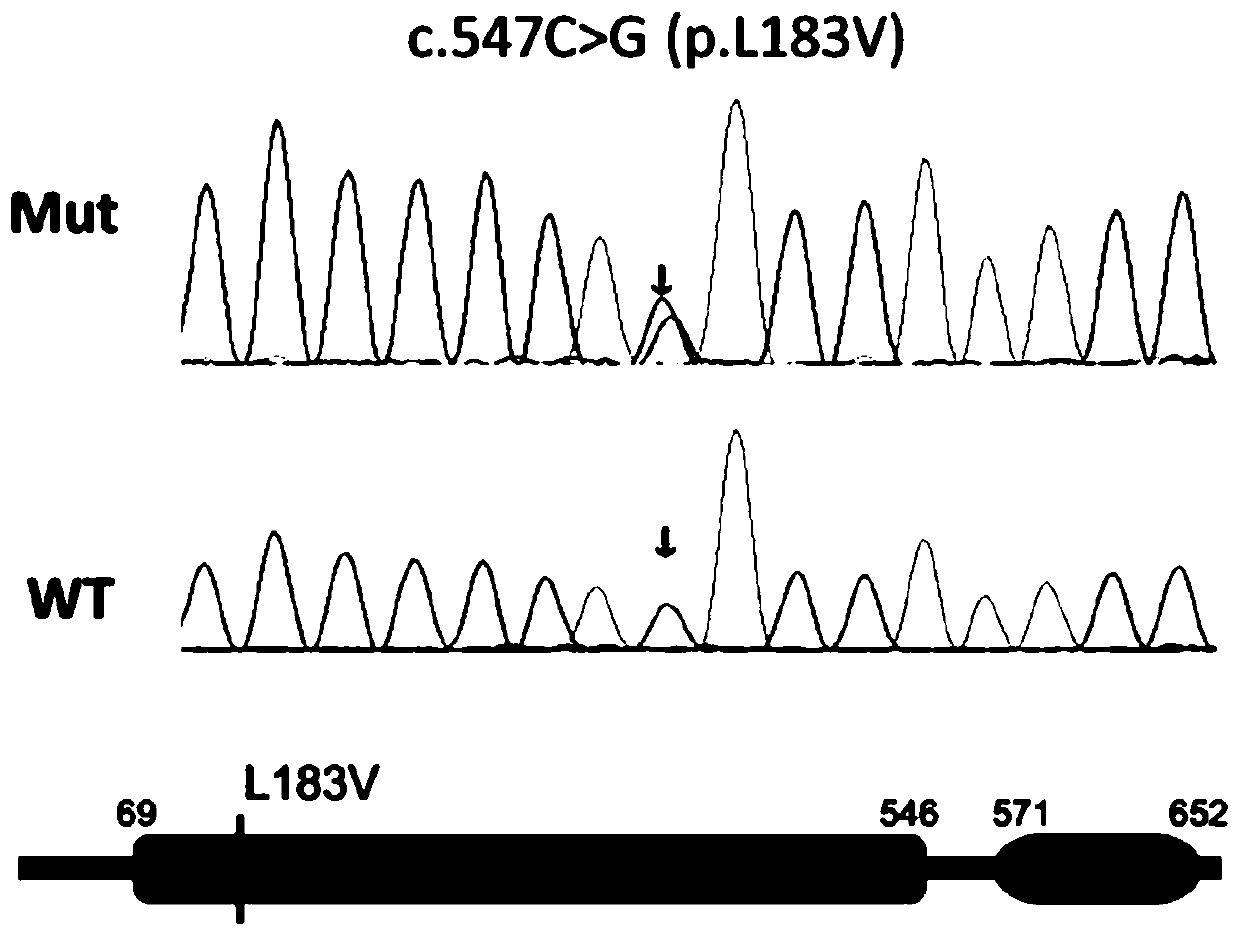
[0056] The sequence of the HPR1 mutant is shown in SEQ ID: NO: 2, and its specific PCR upstream and downstream primers are designed by Primer 5, and Invitrogen Company is responsible for primer synthesis, with a purity of PAGE grade. The synthesized primers are dissolved in RNase free H2O, and The concentration is 10 μM.
[0057] Prepare a kit comprising the following components:
[0058] (a) Extraction system:
[0059] 1) Trizol reagent, 2 tubes, 2000μL / tube;
[0060] 2) Chloroform, 1 tube, 500 μL / tube;
[0061] 3) Absolute ethanol, 1 tube, 8000 μL / tube;
[0062] 4) RNase free ddH 2 O, 2 tubes, 2000 μL / tube;
[0063] 5) Isopropanol, 8000 μL / tube;
[0064] (b) Reverse transcription system:
[0065] 1) Total RNA reverse transcription primer Oligo dT, 1 tube, concentration: 50 μM, 50 μL / tube;
[0066] 2) Reverse transcriptase (200U / μL) 50μL;
[0067] 3) dNTP Mixture (10mM each) 50μL;
[0068] 4) Reverse trans...
Embodiment 3
[0076] Example 3 Sequence detection of HPR1 gene in peripheral blood cells of deaf patients
[0077] After adding TRIzol to peripheral blood cells, place it at room temperature for 10 minutes to fully lyse the samples. Add 200 μl of chloroform to every 1ml of TRIzol, vibrate vigorously and mix well, then place at room temperature for 3-5min to allow natural phase separation. Centrifuge at 12,000 rpm at 4°C for 15 min. The sample will separate into three layers: a yellow organic phase, an intermediate layer and a colorless aqueous phase. The RNA is mainly in the aqueous phase. Transfer the aqueous phase to a new tube. An equal volume of ice-cold isopropanol was added to the supernatant and left at room temperature for 15 min. Centrifuge at 12,000 rpm at 4°C for 10 min, discard the supernatant, and precipitate the RNA at the bottom of the tube. Add 1ml of 75% ethanol to the RNA pellet, shake the centrifuge tube gently, and suspend the pellet. Add 1 ml of 75% ethanol per 1 ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com