Preparation method and application of catalyst for solid acid catalysis of C4 alkylation
A technology of acid-catalyzed carbon and catalyst, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, chemical instrument and method, etc., which can solve the problem of unfavorable separation of alkylated oil products and easy carbon deposition Deactivation, limiting industrial applications and other issues, to achieve the effect of reducing the deactivation rate of catalytic carbon deposition, increasing the hydrogen transfer rate, and increasing the hydrogen transfer rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
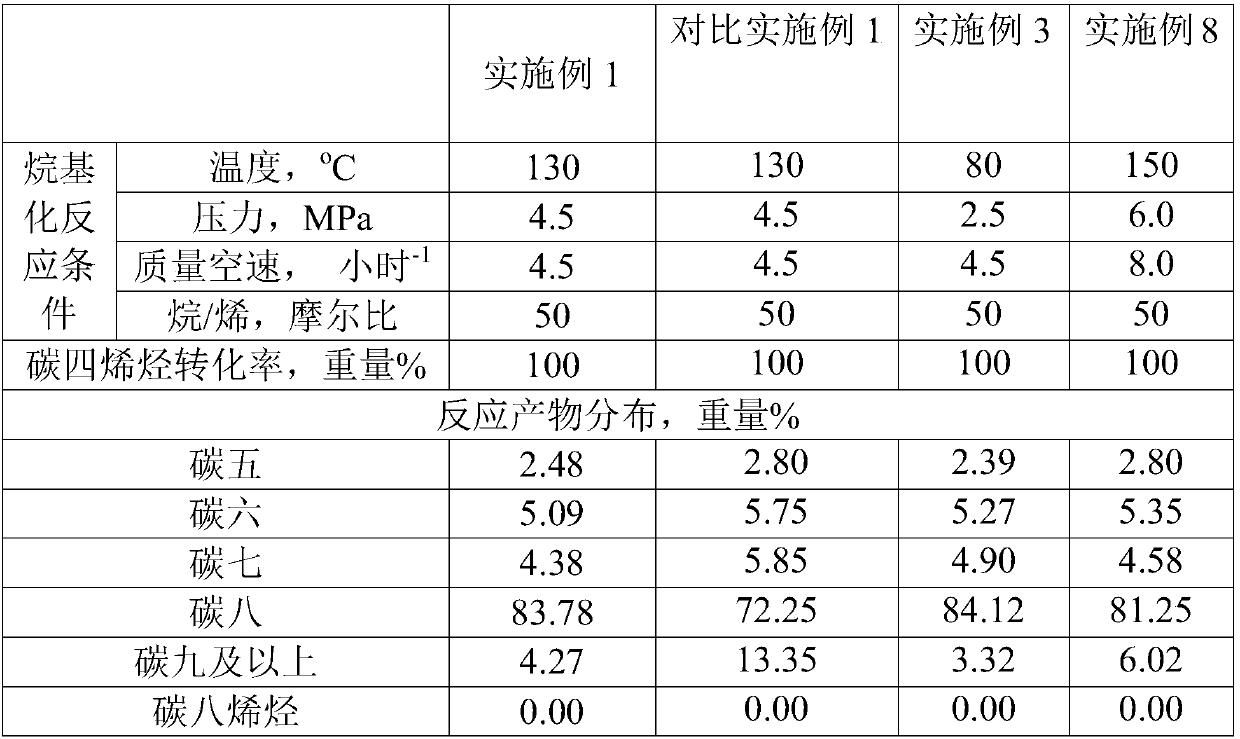
[0018] 50.7mL tetraethyl orthosilicate, 2.5mL hexadecyl long organosilane quaternary ammonium salt ((CH 3 O) 3 SiC 3 h 6 N(CH 3 ) 2 C 16 h 33 Cl) and 91mL of 0.5mol / L tetrapropylammonium hydroxide aqueous solution were added to a 250mL round bottom flask, stirred and hydrolyzed at 35°C for 2h, then added aluminum nitrate according to Si / Al=50, stirred for another 30 minutes, and then pressed 0.5 Add potassium chloroplatinite at wt% load, add iron porphyrin at 2wt% load, add phosphotungstic acid at 10wt% load, continue stirring for 2 hours, and then transfer to a stainless steel crystallization kettle with a polytetrafluoroethylene liner , crystallized at 250°C for 72h, filtered with suction, dried, and calcined at 500°C to remove tetrapropylammonium hydroxide to obtain Pt-Fe-HPA / ZSM-5 catalyst.
Embodiment 2
[0021] Embodiment 2: heteropoly acid type and load
[0022] 50.7mL tetraethyl orthosilicate, 2.5mL hexadecyl long organosilane quaternary ammonium salt ((CH 3 O) 3 SiC 3 h 6 N(CH 3 ) 2 C 16 h 33 Cl) and 91mL of 0.5mol / L tetrapropylammonium hydroxide aqueous solution were added to a 250mL round bottom flask, stirred and hydrolyzed at 35°C for 2h, then added aluminum nitrate according to Si / Al=50, stirred for another 30 minutes, and then pressed 0.5 Add potassium chloroplatinite at wt% load, add iron porphyrin at 2wt% load, add silicotungstic acid at 40wt% load, continue stirring for 2 hours, and then transfer to a stainless steel crystallization kettle with a polytetrafluoroethylene liner , crystallized at 250°C for 72h, filtered with suction, dried, and calcined at 500°C to remove tetrapropylammonium hydroxide to obtain Pt-Fe-HPA / ZSM-5 catalyst.
Embodiment 3
[0023] Embodiment 3: heteropoly acid type and load
[0024] 50.7mL tetraethyl orthosilicate, 2.5mL hexadecyl long organosilane quaternary ammonium salt ((CH 3 O) 3 SiC 3 h 6 N(CH 3 ) 2 C 16 h 33 Cl) and 91mL of 0.5mol / L tetrapropylammonium hydroxide aqueous solution were added to a 250mL round bottom flask, stirred and hydrolyzed at 35°C for 2h, then added aluminum nitrate according to Si / Al=50, stirred for another 30 minutes, and then pressed 0.5 Add potassium chloroplatinite at wt% load, add iron porphyrin at 2wt% load, add germanium tungstic acid at 5wt% load, continue stirring for 2 hours, and then transfer to a stainless steel crystallization kettle with a polytetrafluoroethylene liner , crystallized at 250°C for 72h, filtered with suction, dried, and calcined at 500°C to remove tetrapropylammonium hydroxide to obtain Pt-Fe-HPA / ZSM-5 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com