Dication liquid electrolyte, and preparation and application thereof
A dual-cation, electrolyte additive technology, applied in non-aqueous electrolyte batteries, circuits, electrical components, etc., can solve problems such as the reduction of battery Coulombic efficiency, and achieve the effects of inhibiting reduction deposition, high capacity retention, and improving cycle stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
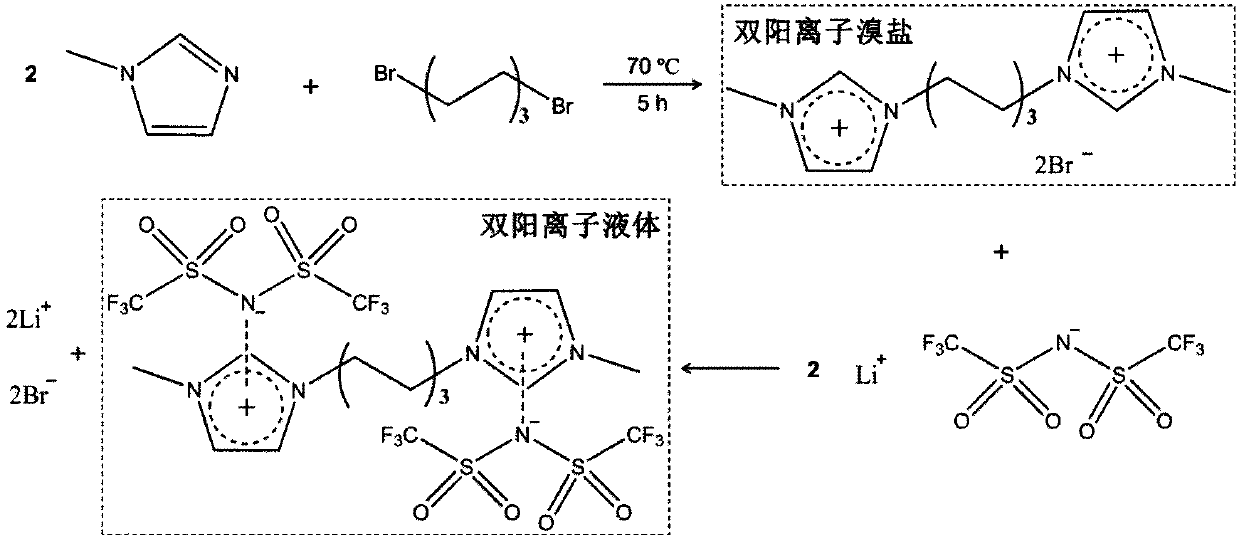
[0039] Weigh 3.3g of N-methylimidazole and 5.0g of 1,6-dibromohexane in a 100mL flask, reflux at 70°C for 5h, add 10mL of deionized water after the temperature drops to room temperature, and filter to obtain the filtrate in a vacuum oven for 120 After drying at ℃ for 48 hours, the white powder was obtained as dication bromide salt with a yield of 98%. Weigh 2.0 g of the obtained dicationic bromide salt and 2.9 g of LiTFSI and dissolve them in 20 mL of deionized water respectively, mix the two solutions slowly, phase separation occurs in the water, use a separatory funnel to obtain the lower liquid, and wash it with deionized water for 3 times , a colorless dianionic liquid was obtained with a yield of 99%.
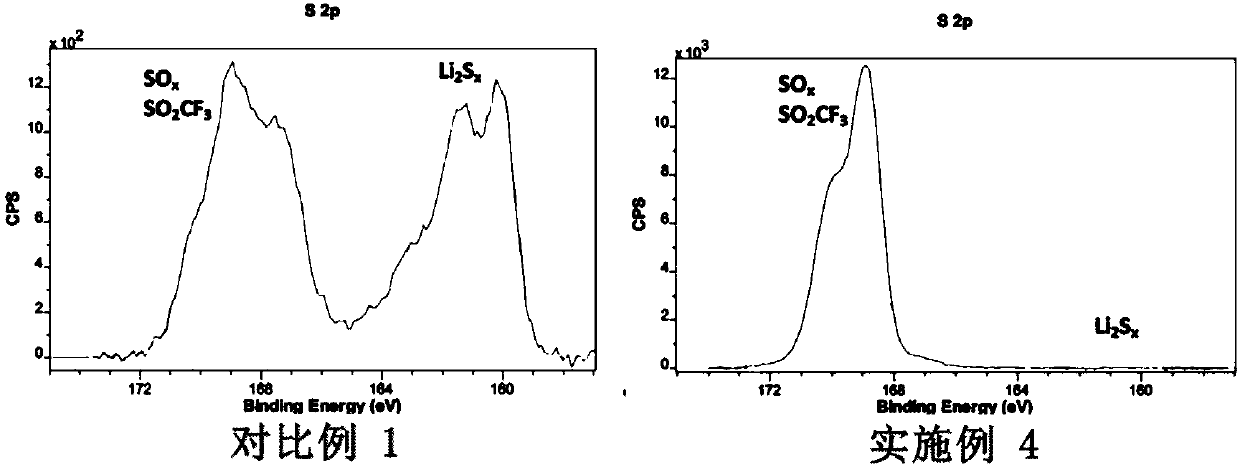
[0040] The diionic liquid was added to the electrolyte solution in Comparative Example 1, and the mass fraction was 2%. The positive electrode was prepared according to the method of Comparative Example 1, the electrolyte solution containing 2wt.% diionic liquid was selec...
Embodiment 2
[0042]According to the method of Example 1, the dicationic liquid was prepared. The diionic liquid was added to the electrolyte solution in Comparative Example 1, and the mass fraction was 5%. The positive electrode was prepared according to the method of Comparative Example 1, the electrolyte solution containing 5wt.% diionic liquid was selected, and the CR2016 button battery was assembled, and the cycle performance charge and discharge test was carried out at a rate of 0.2C.
Embodiment 3
[0044] According to the method of Example 1, the dicationic liquid was prepared. The diionic liquid was added to the electrolyte solution in Comparative Example 1, and the mass fraction was 10%. The positive electrode was prepared according to the method of Comparative Example 1, the electrolyte solution containing 10wt.% diionic liquid was selected, and the CR2016 button battery was assembled, and the cycle performance charge and discharge test was carried out at a rate of 0.2C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com