Copolymer rubber and method for producing same, and crosslinked rubber composition
A manufacturing method and technology for copolymers, applied in the field of cross-linked rubber products, can solve problems such as high reactivity and gelation, and achieve the effects of excellent dispersibility and excellent wear resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0113] Hereinafter, although an Example demonstrates this invention concretely, this invention is not limited to these Examples. In addition, the part in each example is a weight part unless otherwise indicated, and the evaluation of each physical property was performed by the method shown below.
[0114] 1) Molecular weight and molecular weight distribution
[0115] Molecular weight and molecular weight distribution were measured using GPC (manufactured by Tosoh, HLC-8220GPC), using tetrahydrofuran (Tetrahydrofuran, THF) in the solvent, and using a monodisperse polymer at a flow rate of 1.0 ml / min and a column temperature of 38°C. A calibration curve for styrene was used.
[0116] 2) Structure of polyfunctional vinyl aromatic copolymer
[0117] Using the JNM-LA600 nuclear magnetic resonance spectrometer manufactured by JEOL, through 13 C-nuclearmagnetic resonance (NMR) and 1 H-NMR analysis to determine. As a vehicle, use chloroform-d 1 , and the resonance line of tetram...
Synthetic example 1
[0129] 320.5 mL of DVB-810 (manufactured by Nippon Steel & Sumikin Chemicals, with a divinylbenzene content of 81.0% by weight) (1.82 moles of divinylbenzene and 0.43 moles of ethylvinylbenzene), n-butyl acetate 0.28 moles (36.9 mL) and 140 mL of toluene were put into a 1.0 L reactor, and a solution in which 40 millimoles of methanesulfonic acid was dissolved in 0.12 moles (15.7 mL) of n-butyl acetate was added at 70°C to react 6 Hour. After stopping the polymerization solution with calcium hydroxide, it filtered using activated alumina as a filter aid. Thereafter, 22.6 g of a soluble polyfunctional vinyl aromatic copolymer (copolymer X1) was obtained by devolatilization under reduced pressure at 60°C.
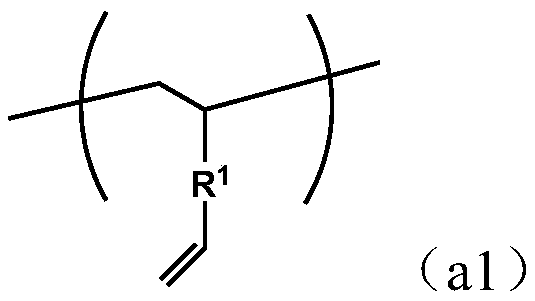
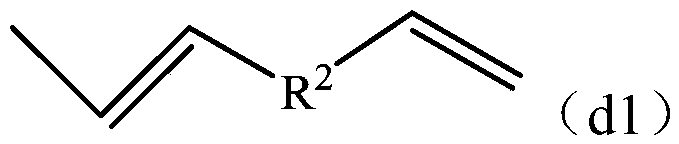
[0130] Mn of the obtained copolymer X1 was 1085, Mw was 12400, and Mw / Mn was 11.4. Through GC analysis, GPC measurement, FT-IR, 13 C-NMR and 1 H-NMR analysis confirmed that the copolymer X has the following vinyl group-containing structural unit (a1) and terminal group (d1...
Synthetic example 2
[0139] Put 276.4 mL of DVB-810, 86.2 mL of toluene, and 93.74 mL of anisole into a 1.0 L reactor, add a solution of 10 mmol of methanesulfonic acid dissolved in 2 mL of toluene at 50°C, and react for 4 hours , except that, the copolymer X2 was obtained by the same method as in Synthesis Example 1.
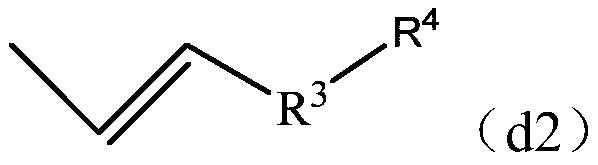
[0140] Mn of the obtained copolymer X2 was 1000, Mw was 18800, and Mw / Mn was 18.8. Through GC analysis, GPC measurement, FT-IR, 13 C-NMR and 1 H-NMR analysis confirmed that the copolymer X2 has the same vinyl group-containing structural unit (a1) derived from the divinyl aromatic compound (a) as in Synthesis Example 1, and the following Terminal group (d3).
[0141] [chemical 9]
[0142]
[0143] (* is the bonding part with the main chain of the copolymer)
[0144] Copolymer X2 contained 64.0 mol % of structural units (a) derived from divinylbenzene components with respect to all structures, and contained 2.0 terminal structural groups (d3) derived from anisole per molecule...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com