Application of ginsenoside CK in preparation of external drugs to treat atopic dermatitis
A technology for atopic dermatitis and ginsenosides, which is applied in the field of medicine, can solve the problems such as few reports on the research effect of atopic dermatitis, and achieve the effects of low recurrence rate, clear active ingredients and stable drug effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0051] Embodiment 1-5 treats atopic dermatitis topical ointment
[0052] Take ginsenoside CK and conventional pharmaceutical carriers according to the weight percentage of the composition in Table 1, prepare according to the conventional preparation method of ointment, and keep it sealed for future use.
Embodiment 6-10
[0053] Example 6-10 Treatment of atopic dermatitis cosmetic health care spray
[0054] Take ginsenoside CK and conventional pharmaceutical carriers according to the weight percentage of the composition in Table 1, prepare according to the conventional preparation method of cosmetic health care spray, and then seal and store for future use.
Embodiment 11
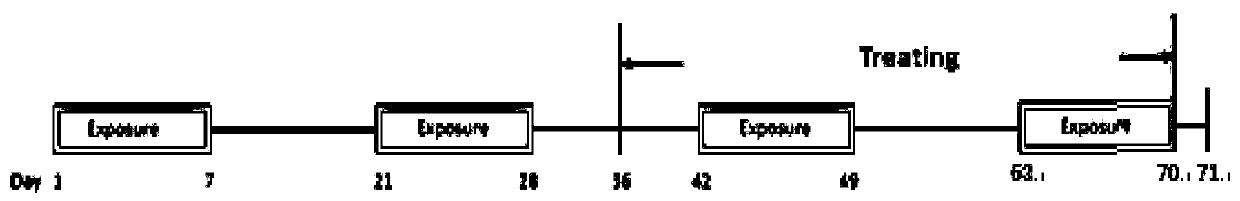
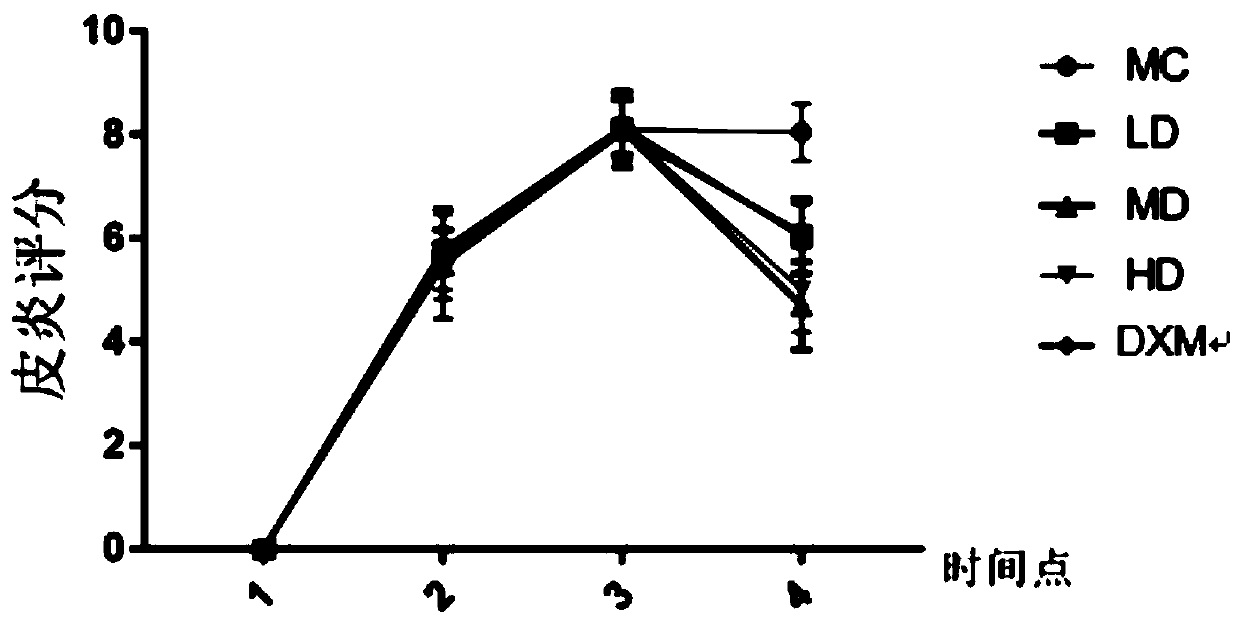
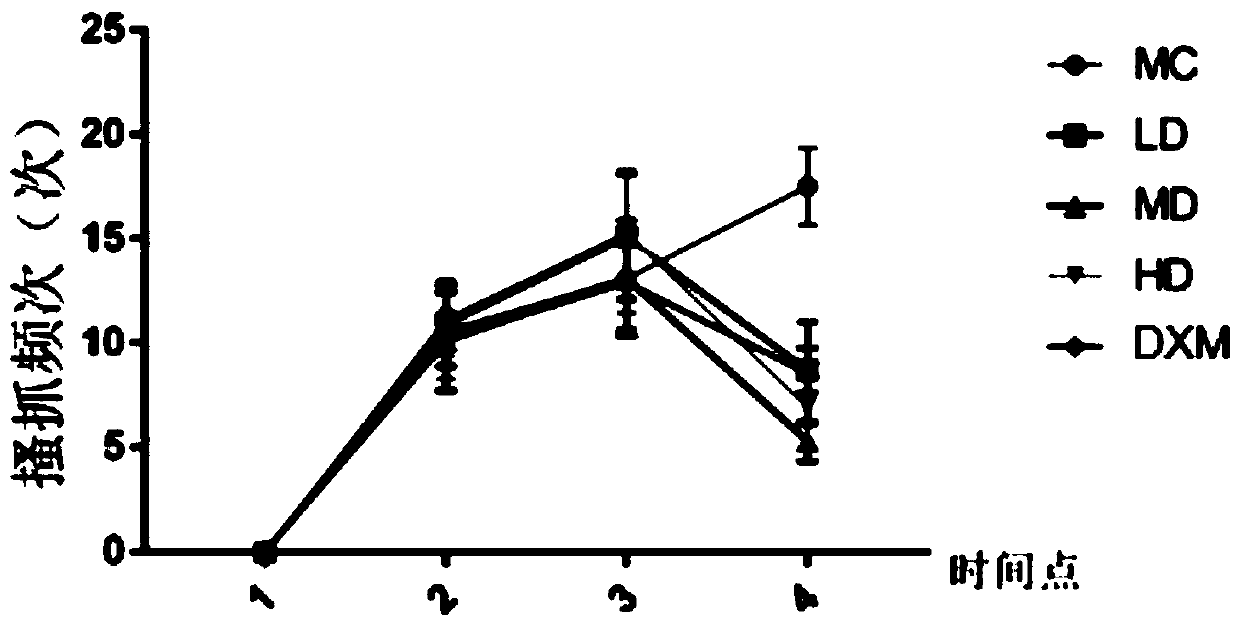
[0055] Embodiment 11 Animal experiments (1)
[0056] In this experiment, a mouse model of atopic dermatitis induced by ovalbumin antigen (OVA) percutaneous sensitization was established and divided into groups, and then ginsenoside CK (low, medium, high concentration) and positive drug (dexamethasone) were used to treat the atopic dermatitis mice. The mice were administered externally, and the therapeutic effects of the mice in each group were observed.
[0057] 1. Experimental materials
[0058] 1. Experimental animals:
[0059] Balb / c mice (female, 6 weeks old, SPF grade, number 60) were kept in the animal room, and the animal room was maintained at a 12:12 hour light-dark cycle, the temperature was 25±2°C, and the humidity 55±15%. Mice were fed with a standard laboratory diet (LabDiet Autoclavable Rodent Diet 5010, PMI International Nutrition, Brentwood, USA), and after two weeks of adaptation, OVA sensitization was performed.
[0060] 2. OVA was purchased from Sigma-Al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com