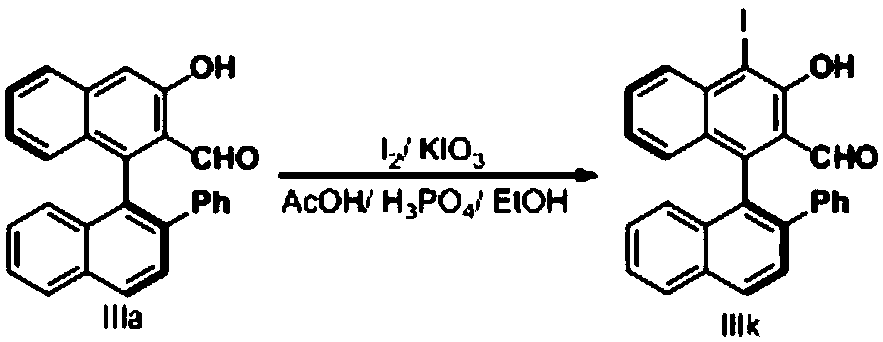Chiral aldehyde catalyst and preparation method thereof, and method for catalyzing asymmetric nucleophilic addition reaction
A catalyst and chiral technology, applied in the field of amino acid compound synthesis, can solve problems such as unfavorable step economy and unfavorable atom economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
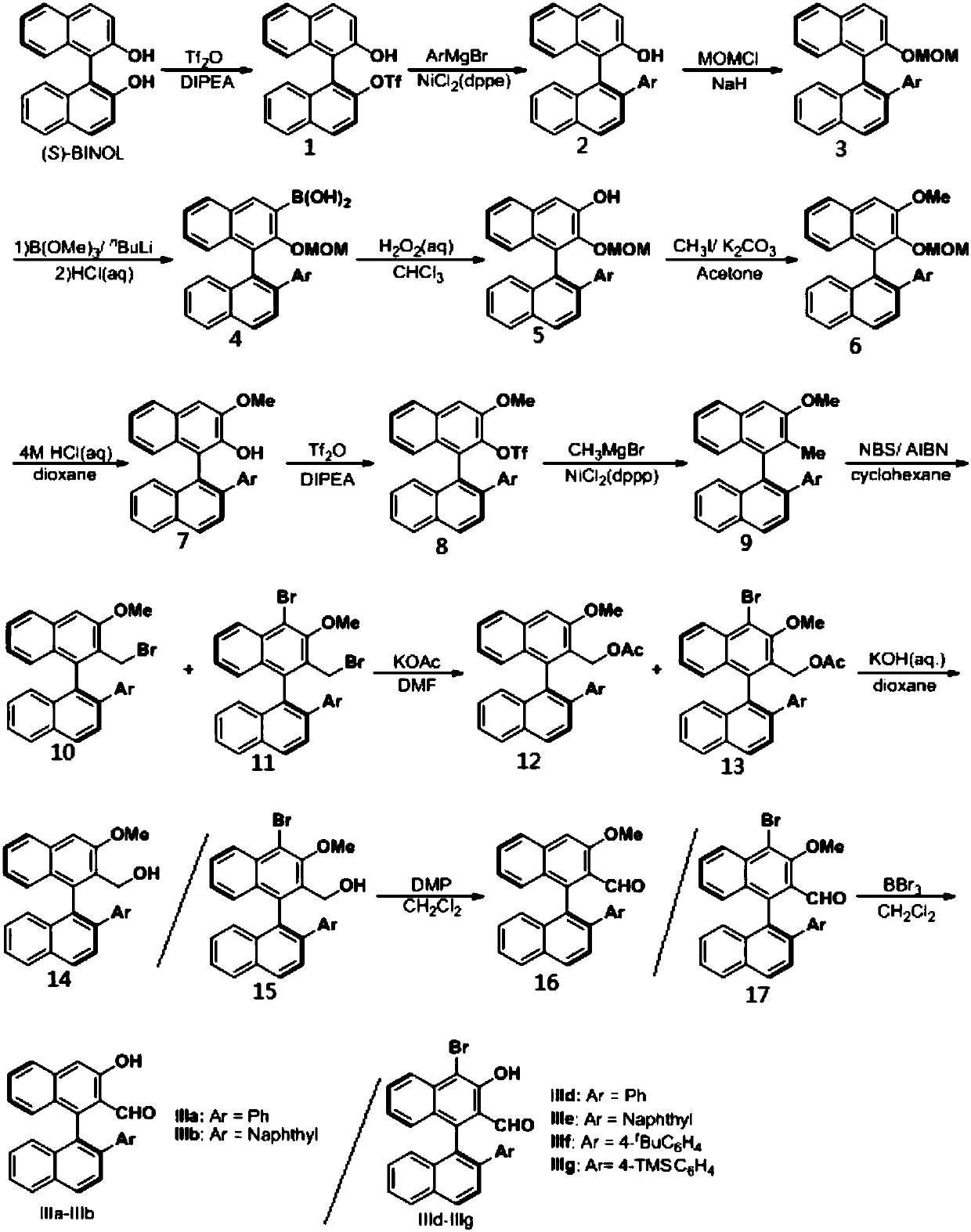
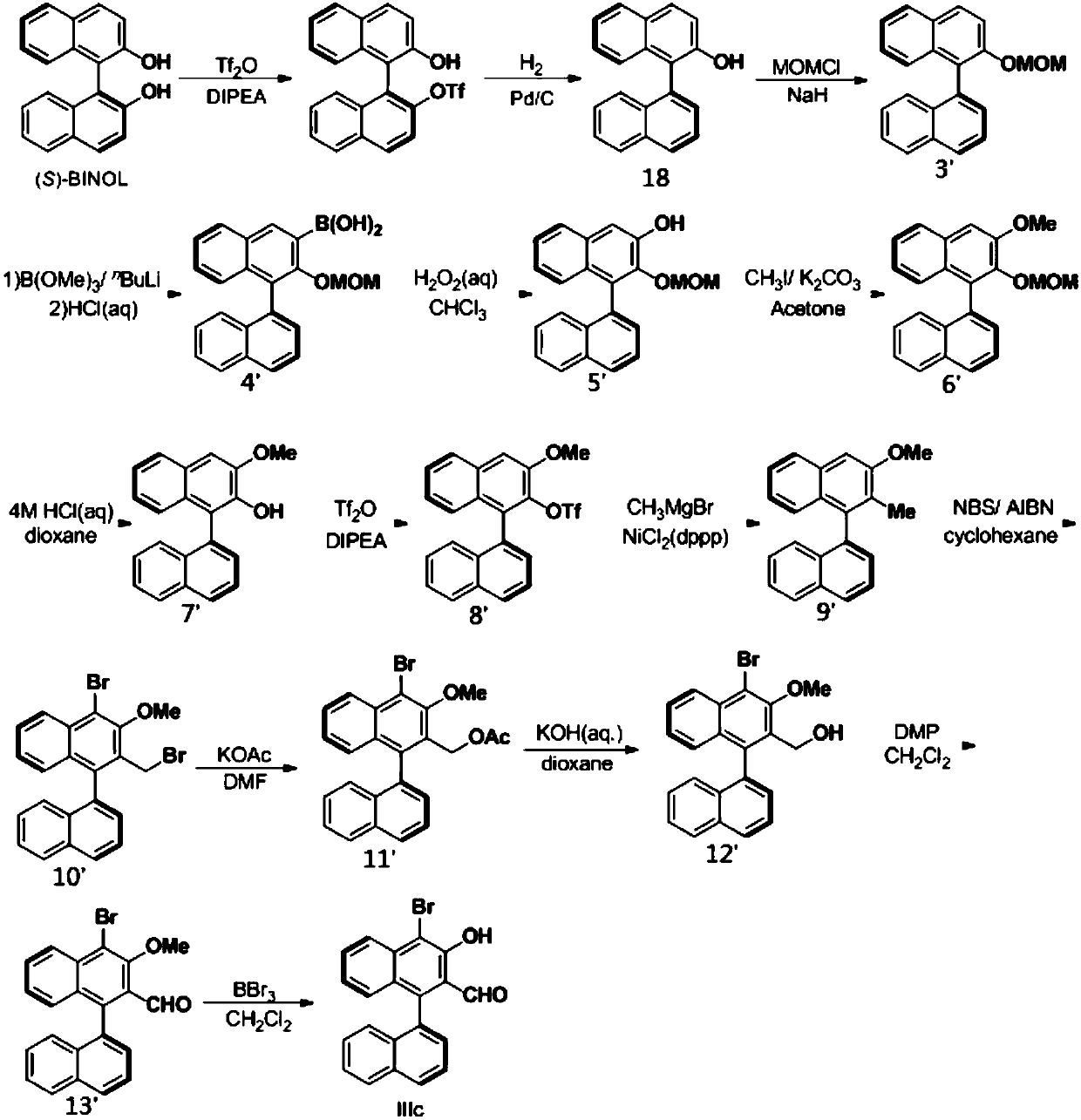
[0056] Embodiment 1, a kind of chiral aldehyde catalyst is prepared according to the following steps:
[0057] Step ①: Dissolve 20mmol (5.72g) of (S)-1,1'-binaphthol ((S)-BINOL) in 50ml of dichloromethane, add 21mmol (3.47ml) of diisopropyl ethyl Base amine (DIPEA), cooled to -78 ° C, added 21mmol (3.44ml) of trifluoromethanesulfonic anhydride (Tf 2 (0), be warmed up to room temperature after stirring for 10min, TLC monitors the reaction, after the reaction is complete, add saturated NaHCO under ice-bath 3 solution, liquid separation to obtain an aqueous phase and an organic phase, the aqueous phase was extracted with dichloromethane, the extract and the organic phase obtained by liquid separation were combined, and anhydrous Na 2 SO 4 After drying the organic phase, the ether was purified by column chromatography (petroleum:AcOEt=30:1) to obtain about 8.04 g of compound 1, with a yield of 96%;
[0058] Step ②: Under nitrogen atmosphere, add 120mmol magnesium chips (2.88g) ...
Embodiment 2
[0101] Example 2, a method for chiral aldehyde catalyzed asymmetric nucleophilic addition reaction of amino acid derivatives, carried out according to the following steps: add α, β-unsaturated carbonyl compound, amino acid ester or amino acid ester hydrochloride to the reaction flask In, any one of the chiral aldehyde catalysts prepared in Example 1, 2,6-dicarboxypyridine, 1,5,7-triazacyclo[4.4.0]dec-5-ene (TBD ), then add 0.5ml of solvent, fully magnetically stirred at 50°C, and monitored by TLC. When the α,β-unsaturated carbonyl compound completely disappears, the entire reaction system is directly spin-dried and passed through the column (the condition of the column is petroleum ether: acetic acid Ethyl ester=10:1, wherein ethyl acetate is a liquid pure substance), obtain proline derivative, in this reaction, the number of carbon atoms of R1 and R2 of α, β-unsaturated carbonyl compound is all less than or equal to 10, amino acid ester Or the number of carbon atoms of R3 and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com