Method for stereoselectively synthesizing beta-2-deoxyglycoside bond
A stereoselective, deoxyglycosidic bond technology, applied in the field of stereoselective synthesis of β-2-deoxyglycosidic bonds, can solve the problems of poor substrate applicability and poor stereoselectivity of β-2-deoxyglycosidic bonds, etc. , to achieve the effect of a wide range of substrates, easy availability of raw materials and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
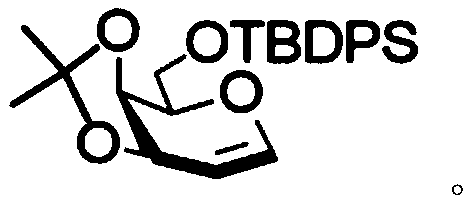
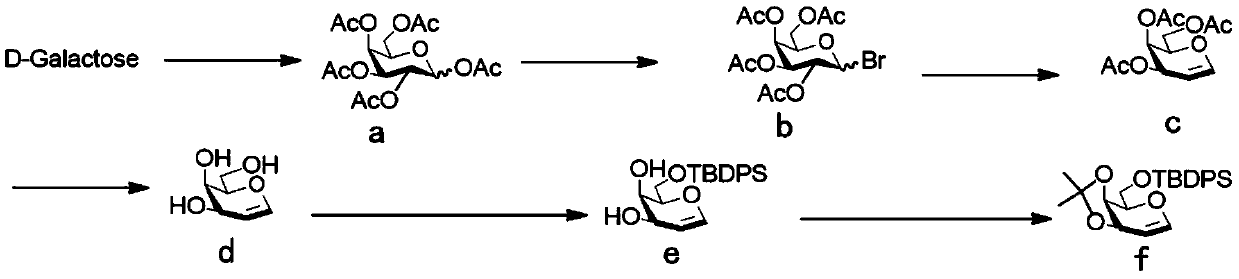
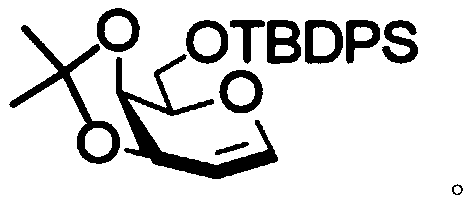
[0041] 3,4-O-isopropylidene-6-O-tert-butyldiphenylsilyl-D-galactose (81 mg, 0.19 mmol), glycosyl acceptor 2,3,4-tri- O-benzyl-α-D-glucopyranoside (134 mg, 0.29 mmol) and freshly activated 4A molecular sieves (220 mg) in dry CH 3 CN:CH 2 Cl 2 (v:v=2:1, 2.9 mL) was cooled to -50 °C. The suspension was stirred for 15 minutes, then NIS (86 mg, 0.38 mmol) and TMSOTf (52 μL, 0.29 mmol) were added. The reaction mixture was stirred at the same temperature for 2 hours. The reaction mixture was gradually warmed to 0 °C and washed with Et 3 N quenching. The mixture was washed with CH 2 Cl 2 dilute and add Na 2 S 2 o 3 , filtered through celite and concentrated in vacuo. The crude material was flash purified by column chromatography (10:1, petroleum ether-EtOAc) to give compound 3,4-O-isopropylidene-6-O-tert-butyldiphenylsilyl-2-deoxy-2 -Iodo-β-D-galactopyranosyl-(1→6)-2,3,4-tri-O-benzyl-α-D-glucopyranoside as a light syrup (159 mg, 82.3%) . R f 0.45 (4:1, petroleumether–E...
Embodiment 2
[0043] 3,4-O-isopropylidene-6-O-tert-butyldiphenylsilyl-D-galactose (52 mg, 0.12 mmol), glycosyl acceptor 2,3,4-tri- O-benzoyl-α-D-mannopyranoside (90 mg, 0.18 mmol) and freshly activated 4A molecular sieves (140 mg) in dry reagent (V CH3CN :V CH2Cl2 =2:1, 1.8mL) and cooled to -50°C. The suspension was stirred for 15 minutes, then NIS (55 mg, 0.24 mmol) and TMSOTf (33 μL, 0.18 mmol) were added. The reaction mixture was stirred at the same temperature for 2 hours. The reaction mixture was gradually warmed to 0 °C and washed with Et 3 N quenching. The mixture was washed with CH 2 Cl 2 dilute and add Na 2 S 2 o 3 , filtered through celite and concentrated in vacuo. The crude material was flash purified by column chromatography (3:1, petroleum ether-EtOAc) to give the product 3,4-O-isopropylidene-6-O-tert-butyldiphenylsilyl-2-deoxy-2 -Iodo-β-D-galactopyranose-(1→6)-2,3,4-tri-O-benzoyl-α-D-mannoside (110 mg, 85.1%). R f 0.4 (petroleum ether:ethyl acetate=3:1). 1 H ...
Embodiment 3
[0045] 3,4-O-isopropylidene-6-O-tert-butyldiphenylsilyl-D-galactose (49 mg, 0.12 mmol), glycosyl acceptor 2,3,4-tri- O-benzyl-α-D-galactopyranoside (80 mg, 0.17 mmol) and freshly activated 4A molecular sieves (130 mg) in dry reagent (V CH3CN :V CH2Cl2 =2:1, 1.7mL) and cooled to -50°C. The suspension was stirred for 15 minutes, then NIS (52 mg, 0.23 mmol) and TMSOTf (31 μL, 0.17 mmol) were added. The reaction mixture was stirred at the same temperature for 2 hours. The reaction mixture was gradually warmed to 0 °C and washed with Et 3 N quenching. The mixture was washed with CH 2 Cl 2 dilute and add Na 2 S 2 o 3 , filtered through celite and concentrated in vacuo. The crude material was flash purified by column chromatography (3:1, petroleum ether-EtOAc) to give the product 3,4-O-isopropylidene-6-O-tert-butyldiphenylsilyl-2-deoxy-2 -Iodo-β-D-galactopyranose-(1→6)-2,3,4-tri-O-benzyl-α-D-galactoside (102 mg, 87.4%). R f 0.4 (petroleum ether:ethyl acetate=3:1). 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com