Method for preparing D-p-methylsulfonylphenylserine ethyl ester with high stereoselectivity
A methylsulfonyl phenylserine ethyl ester and stereoselectivity technology, which is applied in the field of organic chemistry of small molecule catalysis, can solve the problems of low product yield, low yield, increased cost and the like, and achieves good stereoselectivity and reaction conditions. Gentle, recycling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1000g p-thymphenyl benzaldehyde is added to the reactor containing 10L dichloromethane, and PS-L-proline is added (L-proline load is 0.9mmol / g, 5mol%, in terms of proline) , 1100g N-Boc-glycine ethyl ester and di-n-butyl boron trifluoromethanesulfonate (2978g), after reacting at 25°C for 30 hours, realize the separation of the catalyst and the reaction system by means of suction filtration, and then to the reaction 1L of trifluoroacetic acid was added to the system for 1.5 hours, and then the reaction solvent was removed in vacuo. The obtained crude product was purified by recrystallization. The recrystallization solvent was a mixed solvent of dichloromethane and n-hexane (volume ratio: 1:1), The recrystallization temperature is room temperature, and D(-)-p-MPSE can be obtained with a yield of 45%.
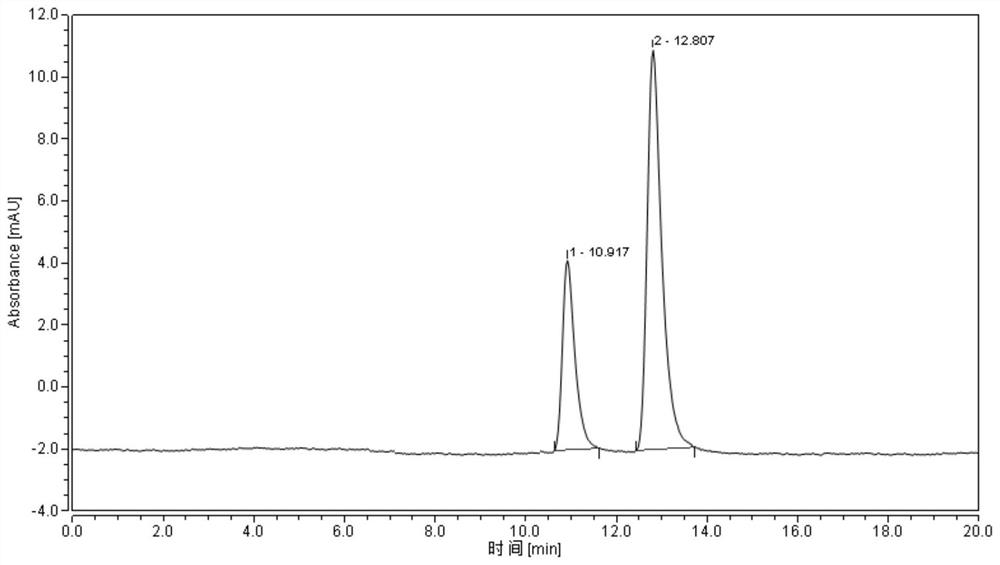
[0036] Purity detection: the optical purity of the product of the present invention is detected by high performance liquid chromatography, and the results are as follows: ...
Embodiment 2
[0039] 1000g p-thymphenicyl benzaldehyde is added in the reactor that fills 10L methylene dichloride, the added PS-L-proline (L-proline load is 0.9mmol / g, 10mol%, calculated as proline ), 1100g N-Boc-glycine ethyl ester and di-n-butyl boron trifluoromethanesulfonate (2978g), after reacting for 30 hours at 25°C, the catalyst was separated from the reaction system by means of suction filtration. 1L trifluoroacetic acid was added to the system for 1.5 hours, and then the reaction solvent was removed in vacuo. The obtained crude product was purified by recrystallization. The recrystallization solvent was a mixed solvent of dichloromethane and n-hexane (volume ratio: 1:1). D(-)-p-MPSE can be obtained with a yield of 56%.
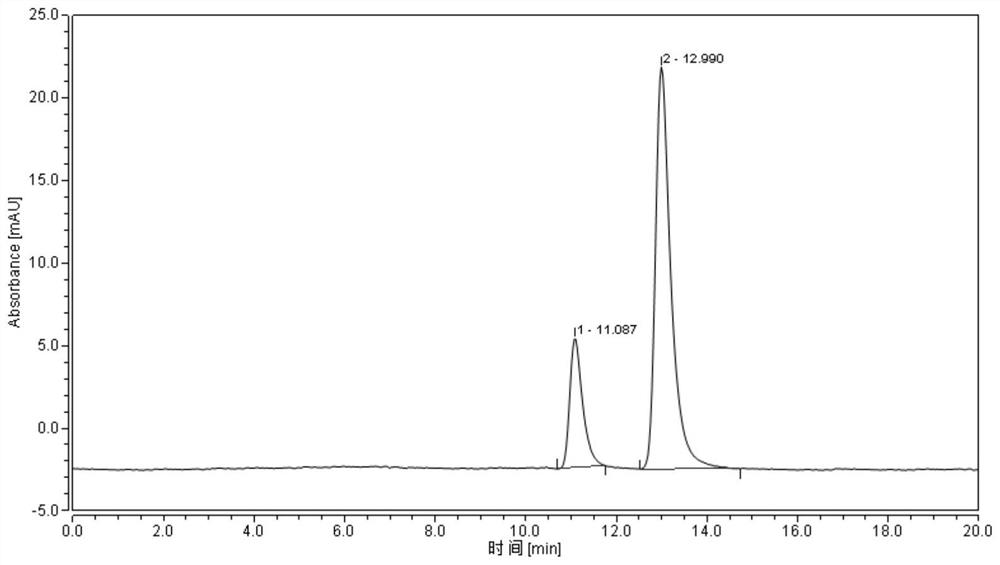
[0040] Purity detection: the optical purity of the product of the present invention is detected by high performance liquid chromatography, and the results are as follows: figure 2 Shown, show that the ee value of obtained product is 59.94%, and the retention ti...
Embodiment 3
[0043]1000g p-thymphenicyl benzaldehyde is added in the reactor that fills 10L methylene dichloride, the added PS-L-proline (L-proline load is 0.9mmol / g, 20mol%, calculated as proline ), 1100g N-Boc-glycine ethyl ester and di-n-butyl boron trifluoromethanesulfonate (2978g), after reacting for 30 hours at 25°C, the catalyst was separated from the reaction system by means of suction filtration. 1L trifluoroacetic acid was added to the system for 1.5 hours, and then the reaction solvent was removed in vacuo. The obtained crude product was purified by recrystallization. The recrystallization solvent was a mixed solvent of dichloromethane and n-hexane (volume ratio: 1:1). D(-)-p-MPSE can be obtained with a yield of 73%.
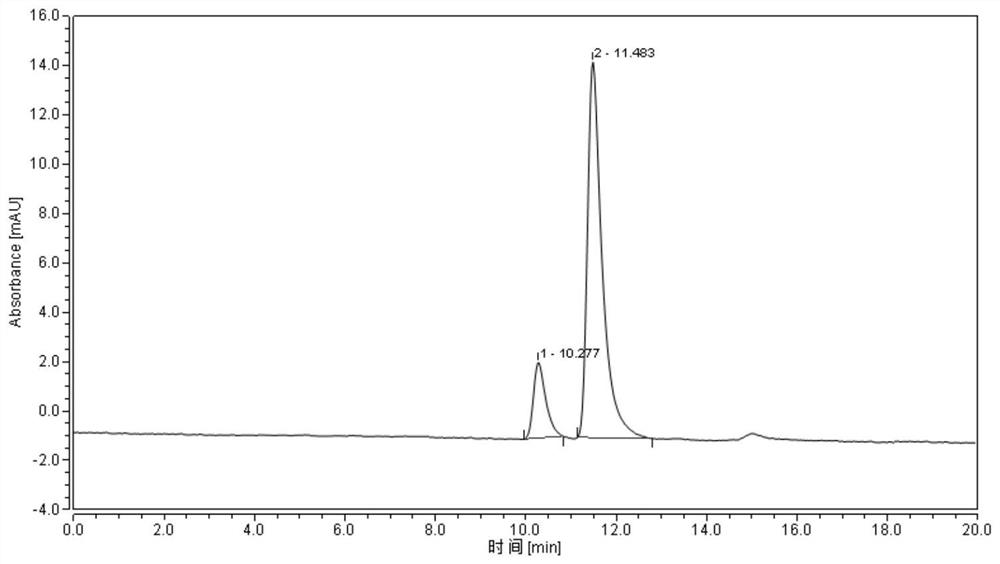
[0044] Purity detection: the optical purity of the product of the present invention is detected by high performance liquid chromatography, and the results are as follows: image 3 Shown, show that the ee value of obtained product is 71.24%, and the retention time...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com