Preparation method for semaglutide intermediate
A technology of semaglutide and side chain, applied in the field of pharmaceutical chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
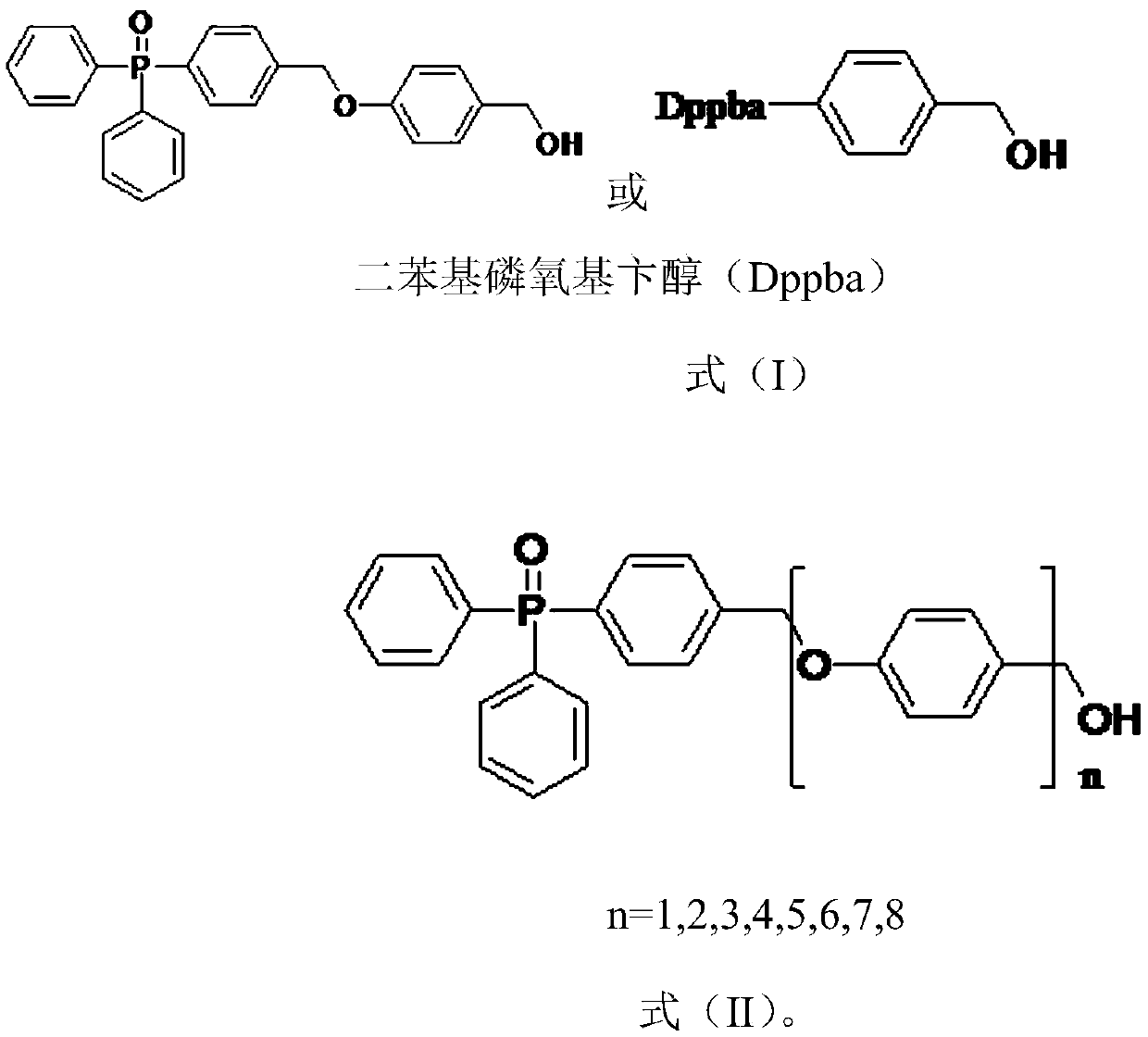
[0068] A protective group compound 1 and compound 1 analogs synthesized by a semaglutide side chain of the present invention are shown in formula (I) and formula (II) respectively:
[0069]
[0070] Formula (I)
[0071]
[0072] n=1,2,3,4,5,6,7,8
[0073] Formula (II).
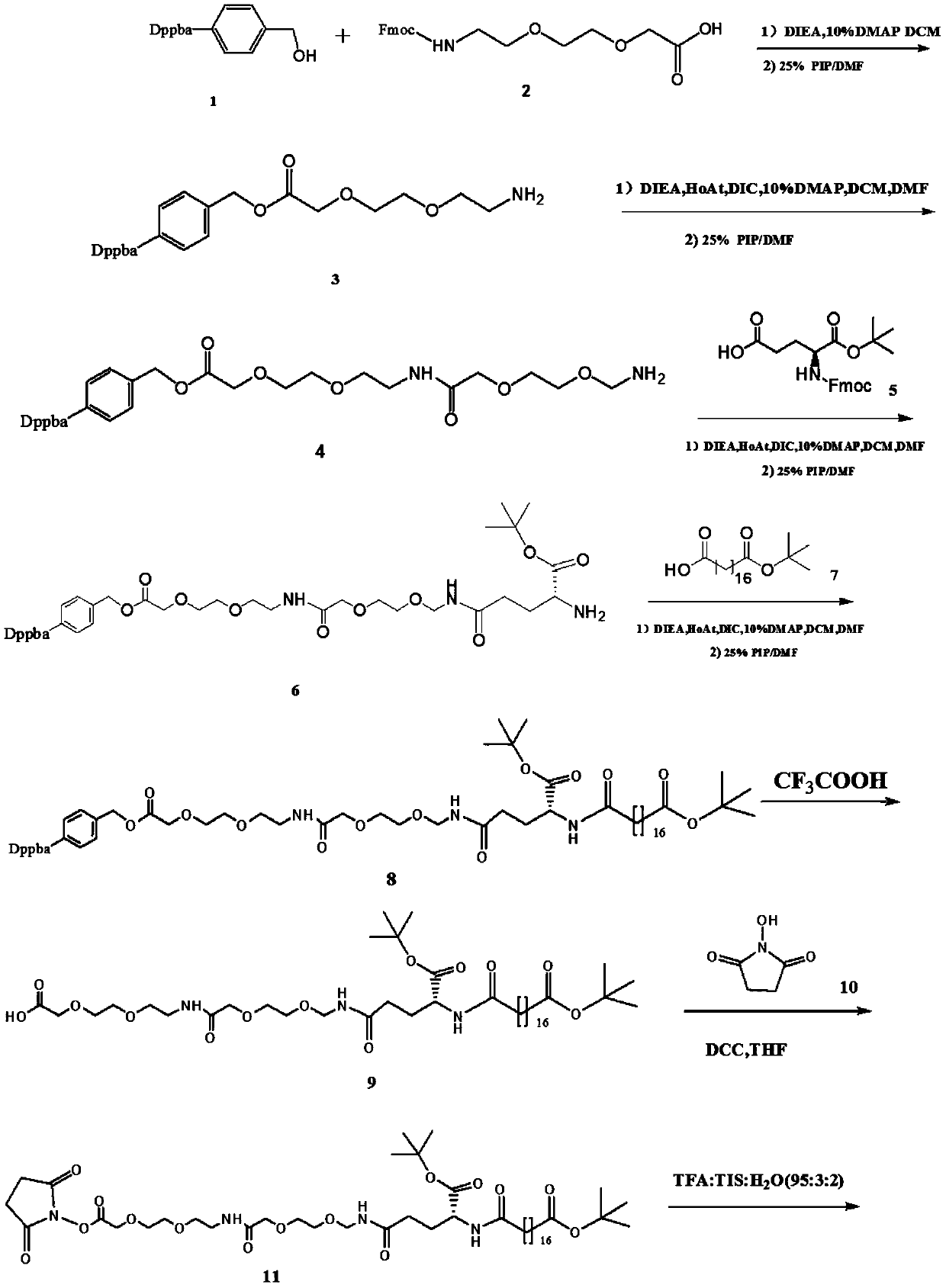
[0074] The preparation method of the protective group compound 1 and the compound 1 analog of a kind of semaglutide side chain synthesis of the present invention comprises the following steps: dissolving diphenylphosphoxybenzyl alcohol (0.26mol) in 400mL and drying To the tetrahydrofuran solution, the temperature was lowered to -5°C, sodium hydrogen (0.39mol) was added in batches, and after stirring for 50 minutes under ice bath, a dry tetrahydrofuran solution (100mL) of p-chlorobenzyl alcohol (50.7g, 0.26mol) was added dropwise , keep the internal temperature less than 5 degrees; after dropping, stir overnight at room temperature, carefully drop saturated ammonium chloride solution in an ice bath unti...
Embodiment 2
[0106] The difference between Example 2 and Example 1 is that a method for preparing a semaglutide side chain of the present invention comprises the following steps:
[0107] In step (E), the synthesis of compound 8
[0108] Accurately weigh 265mg HOAt in a 25ml beaker, add 300ul DIC, then add 312mg of compound 7 in DMF:DCM (volume ratio 4:1, 10ml) solution, mix for 15min, then add 420mg of DIEA; add the mixture to CH obtained above 2 Cl 2 Layer (after drying), mixed reaction at room temperature for 1h, ninhydrin detection reaction progress;
[0109] The reaction mixture was washed with saturated NH 4 Cl(aq) was washed twice, followed by saturated Na 2 CO 3 (aq) Washed twice, the combined organic layers were washed with MgSO 4 Drying, filtration and evacuation afforded the crude protected amino acid by dissolving the crude mixture in a minimum amount of ethyl acetate followed by precipitation with petroleum ether and filtering the resulting white precipitate;
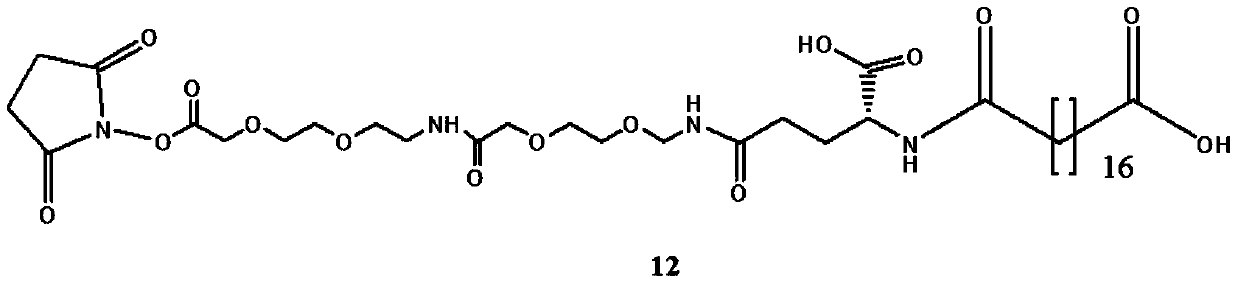
[0110] A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com