Multifunctional fluorescent probe having ESIPT and AIE properties and preparation method and application thereof
A fluorescent probe, multi-functional technology, applied in chemical instruments and methods, fluorescence/phosphorescence, organic chemistry, etc., can solve the problems of complex synthesis process, unfavorable environmental protection, poor solubility, etc., and achieve simple synthesis method and good sensitivity , the effect of simplifying the recognition process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0036] (1) The reaction formula of the synthetic relay type multifunctional fluorescent probe:
[0037]
[0038] (2) Concrete steps for synthesizing relay-type multifunctional fluorescent probes:
[0039] Weigh 0.185g of 2-pyridinecarboxylic acid and dissolve it in 15mL of dry dichloromethane, add 0.682g of benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU) and stir for 30 minutes, then added 0.226g 2-(2′-aminophenyl)benzothiazole and 0.181g triethylamine, and stirred at 25°C for 12h. After the reaction was completed, the solvent was distilled off under reduced pressure, and the residue was separated by column chromatography with the eluent of ethyl acetate and petroleum ether at a volume ratio of 1:10 to obtain a relay-type multifunctional fluorescent probe N-(2-(2 '-Benzothiazolyl)phenyl)pyridinamide, yield 90%. Melting point: 165.5-166.1°C.
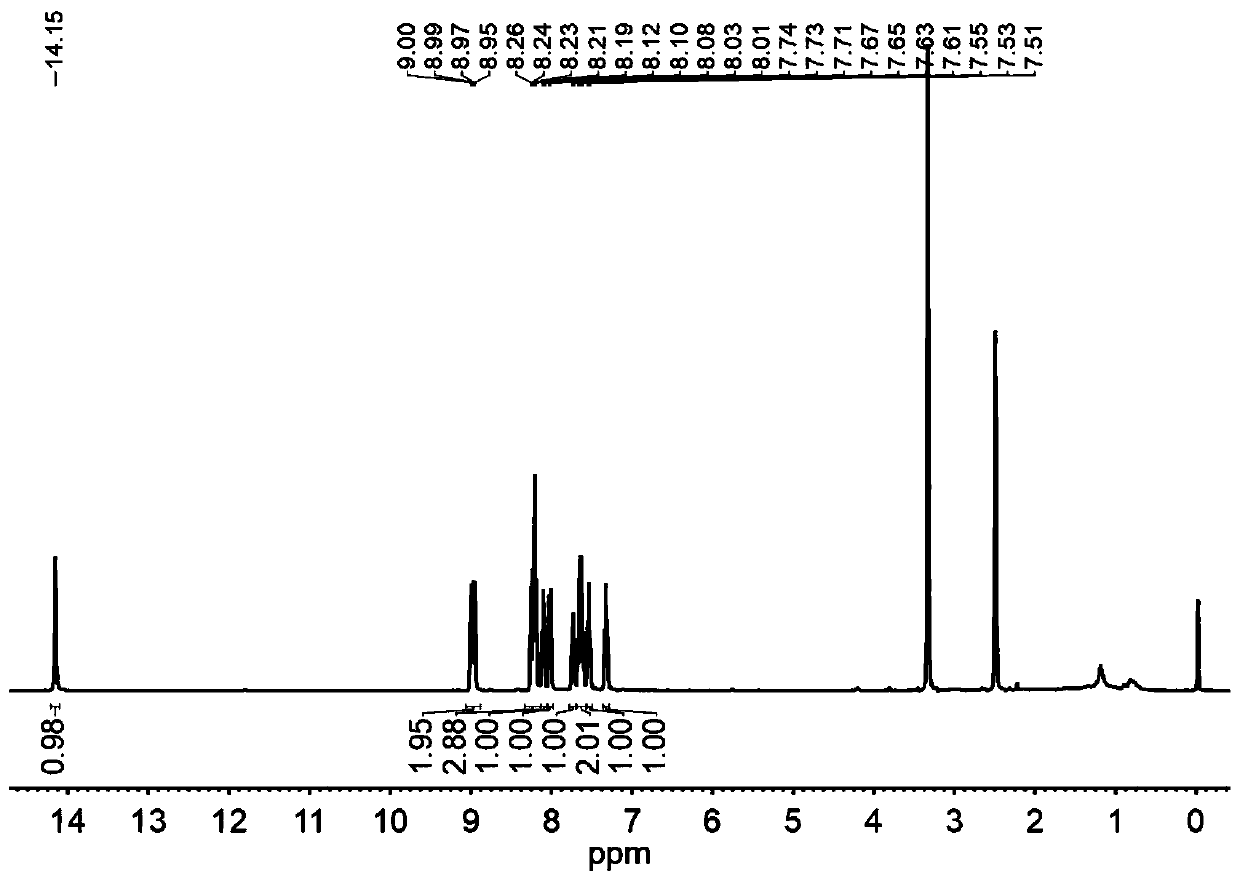
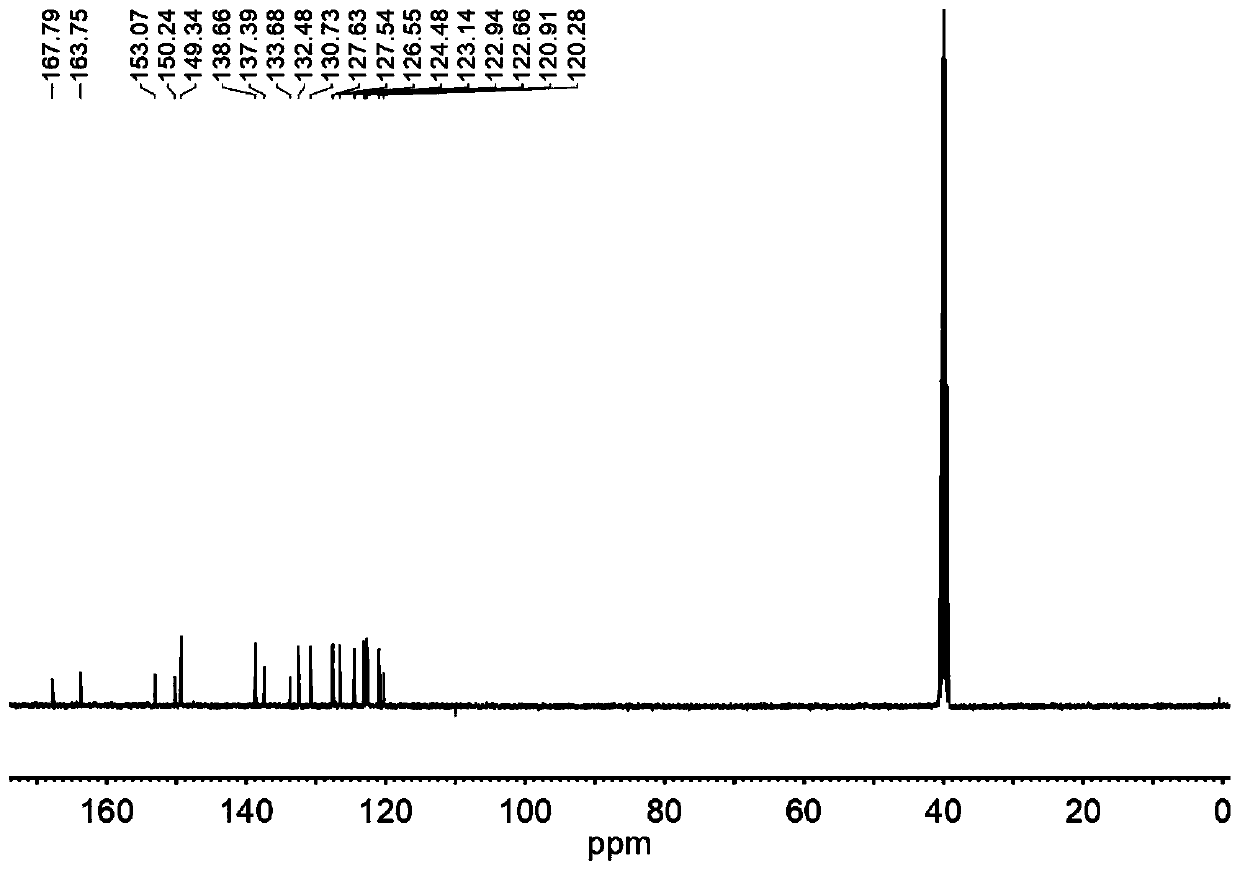
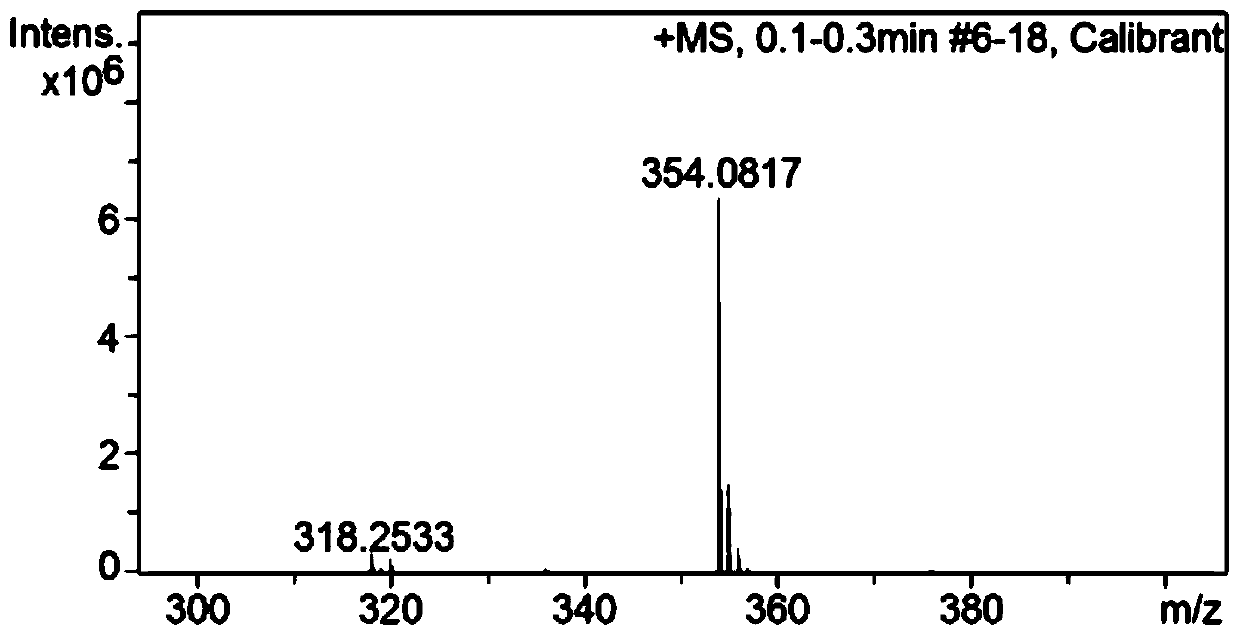
[0040] The results of NMR analysis are as Figure 1-3 Shown: 1 H NMR (400MHz, DMSO-d 6 )δ14.15(s,1H),8.98...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com