A method for ultrasensitive detection of dopamine based on nucleic acid aptamer
A nucleic acid aptamer and sensitive detection technology, applied in the field of biomedicine, can solve the problems of large trauma, poor sensitivity, and complicated operation, and achieve the effects of reducing burden, low cost and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
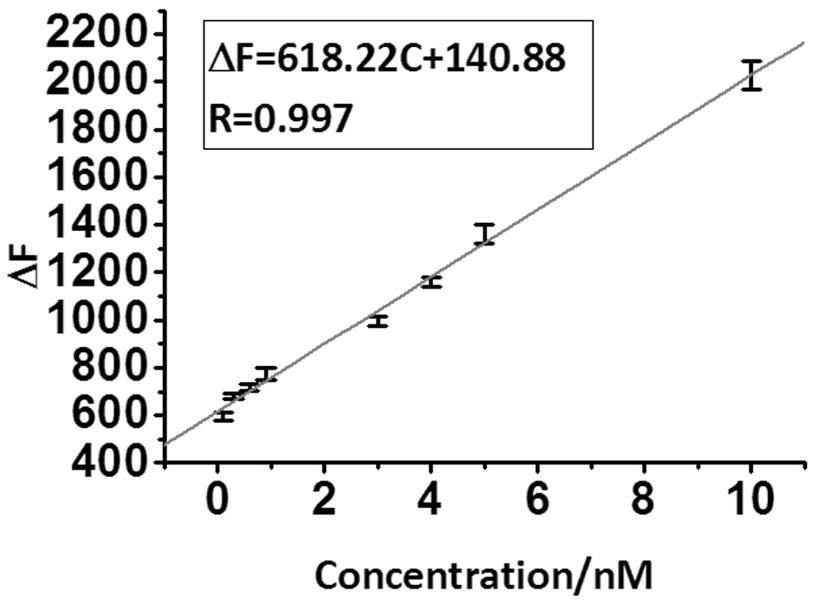
[0022] Embodiment 1 The drawing of standard curve
[0023] (a) Prepare dopamine standard solutions with concentrations of 0nM, 0.1nM, 0.3nM, 0.6nM, 0.9nM, 3.0nM, 4.0nM, 5.0nM and 10.0nM respectively.
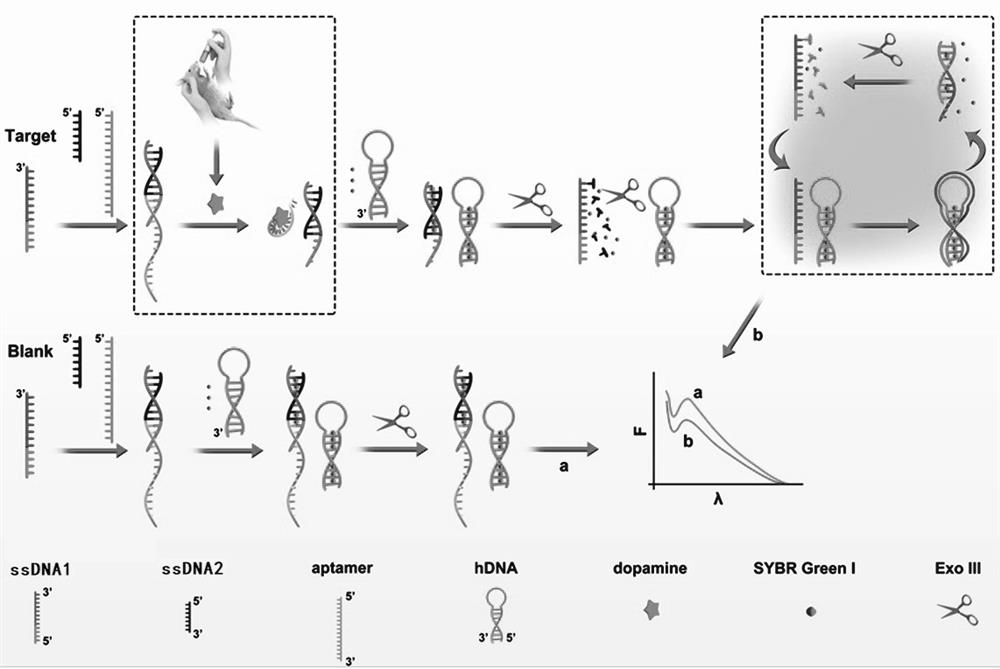
[0024] (b) According to the concentration gradient of the dopamine solution, the detection test was performed in groups, and the test operation in each group was the same, specifically: 6 μL ssDNA1 (5 μM), 6 μL ssDNA2 (5 μM), 6 μL dopamine aptamer (5 μM ) was added to 230 μL Tris-HCl (10 mM) and incubated at room temperature for 5 hours; 20 μL of corresponding concentration of dopamine solution was added to react at room temperature for 1 hour, and 6 μL of hairpin DNA (15 μM) and 19 μL of SYBR Green Ⅰ (purchased from Sangon Bioengineering (Shanghai) Co., Ltd., diluted 1000 times with water) reacted at room temperature for 45 minutes; then added 70U exonuclease III (Exo-Ш) and incubated at 37°C for 1 hour (this was verified by polyacrylamide gel electrophoresis) Enzyme digestion...
Embodiment 2
[0028] Example 2 Detection of Dopamine Content in Mouse Brain Tissue
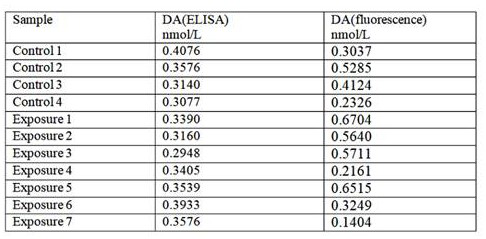
[0029] The mice were anesthetized by intraperitoneal injection of 1% pentobarbital sodium, and the anesthesia dose was 0.1ml / 10g. After complete anesthesia, the brain tissue was collected under low temperature conditions. Add tissue lysate to the brain tissue according to 1g:7.5mL, homogenize for 30s, centrifuge at low temperature (14000r / min, 4°C), take the supernatant and centrifuge again under the same conditions. Fluorescence detection was performed on the supernatant according to the detection steps in Example 1, and enzyme-linked immunosorbent assay was performed according to conventional methods. The test results are shown in Table 1. After statistical analysis, P=0.235>0.1, the difference between the two methods is not statistically significant, so it is considered that the two methods have good consistency.
[0030] Table 1:
[0031]
[0032]The sensitivity of the detection method of the prese...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com