A pharmaceutical composition for resisting cerebral ischemia-reperfusion injury and its application
A composition and drug technology, applied in the field of pharmaceutical compositions against cerebral ischemia-reperfusion injury, can solve the problems of unstable quality, complex and complex components, and difficult to clarify the mechanism of efficacy, so as to restore brain function and reduce cell apoptosis. , Enhanced effect of anti-cerebral ischemia-reperfusion injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Isolation, cultivation and identification of primary rat brain microvascular endothelial cells (rBMECs)
[0034] 1. Preparation of reagents
[0035] (1) 20% serum complete medium: Add 5mL penicillin-streptomycin solution and 100mL fetal bovine serum to 400mL DMEM / F12 medium, shake well, aliquot into 50mL centrifuge tubes, and store in a 4°C refrigerator for later use.
[0036] (2) 0.1% type II collagenase solution: add 20mL DMEM / F12 medium to 100mg type II collagenase powder to make a 0.5% type II collagenase mother solution, pass through a 0.22μm filter membrane to filter and sterilize, and absorb 5mL , add 20mL DMEM / F12 medium, mix well to obtain 0.1% type II collagenase solution, dispense it into 15mL, and store it in a -20°C refrigerator for later use.
[0037] (3) 25% bovine serum albumin (BSA) solution: transfer 10g of BSA powder to a 50mL centrifuge tube, add 40mL of DMEM / F12 basic medium, shake to dissolve slowly, pass through a 0.22μm microporous mem...
Embodiment 2
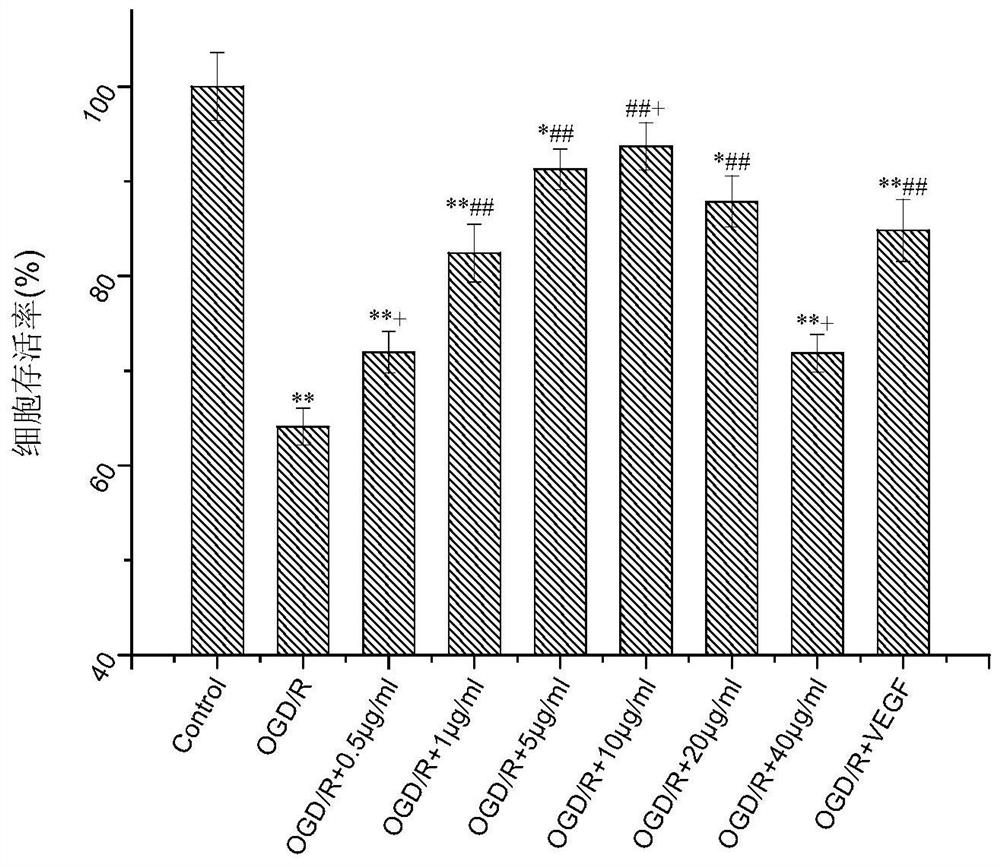
[0059] Example 2 Effects of three active ingredients on the activity of oxygen-glucose deprived brain microvascular endothelial cells
[0060] 1. Preparation of a series of dosing solutions
[0061] Weigh 2mg standard substances of verbascoside, formononetin, and 7-2'-dihydroxy-3',4'-dimethoxyisoflavane respectively, add them to 0.2mL EP tube, and add 50μL DMSO to carry out Dissolved, prepared as 40mg / mL mother solution, and diluted with complete medium to 0.1, 0.5, 1, 5, 10, 20, 40 μg / mL serial administration solutions (DMSO concentration controlled below 0.1%).
[0062] 2. Preparation of VEGF solution
[0063] Take 10μg VEGF and dissolve it in 10mL PBS solution, the concentration is 1μg / mL at this time, and distribute it for use. Pipette 10 μL of the above solution into a sterilized EP tube, and then add 990 μL of complete medium, and the concentration at this time is 10 ng / μL.
[0064] 3. Cell inoculation
[0065] Cells at 25cm 2 When cultured in the culture flask to 8...
Embodiment 3
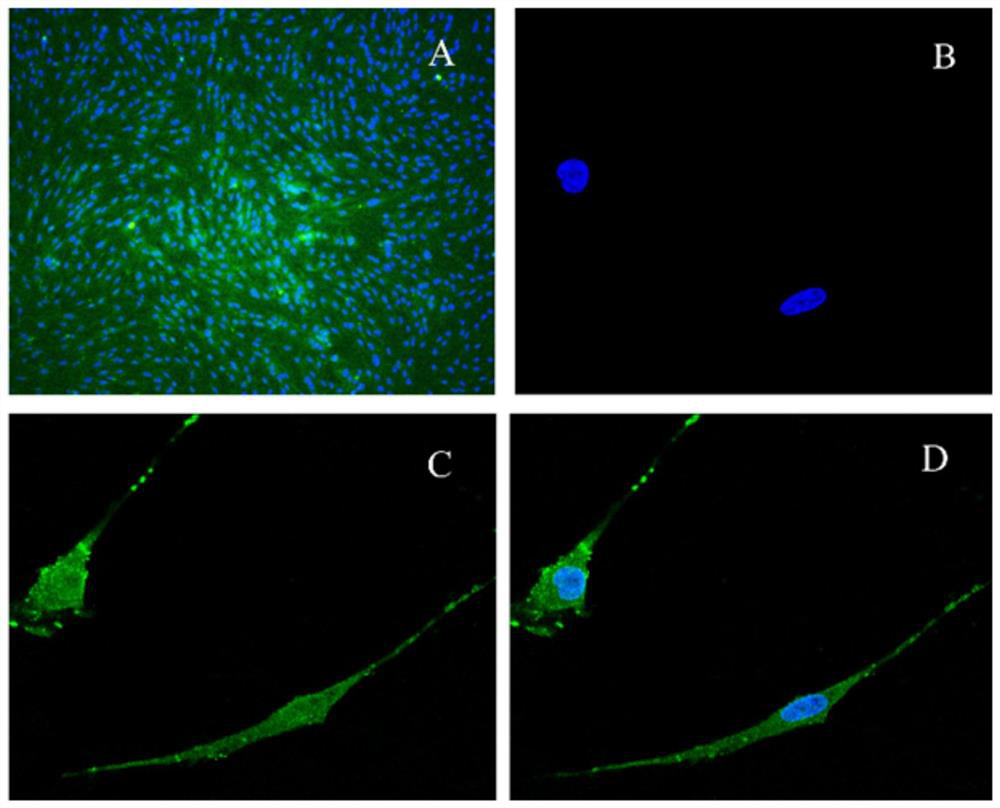
[0083] Example 3 Effects of Active Ingredient Combination on the Expression of Cerebral Microvascular Endothelial Cells VEGF, bFGF, LDH and SOD
[0084] 1. Extraction and separation of primary rBMECs
[0085] Refer to the extraction and separation of primary rBMECs in Step 2 of Example 1.
[0086] 2. Subculture of cells
[0087] Referring to Step 3 of Example 1, the cells were subcultured.
[0088] 3. Cell inoculation
[0089] Refer to Step 3 of Example 2 for cell inoculation.
[0090] 4. Establishment of hypoxic injury model of rat cerebrovascular endothelial cells
[0091] Referring to step 4 of Example 2, the experimental grouping (2) construction of the model group.
[0092] 5. Grouping
[0093] According to the results of the CCK-8 cytotoxicity test, the concentrations of calycosin, formononetin, and 7-2'-dihydroxy-3',4'-dimethoxyisoflavane were in the range of 1-20, 0.1-10, 1-20 μg / mL is a non-cytotoxic dose, that is, after 24 hours of co-culture of each drug and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com