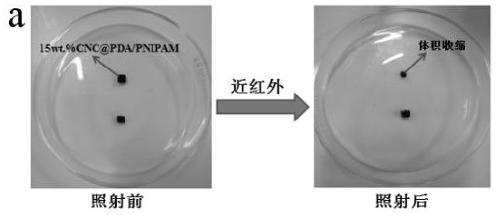
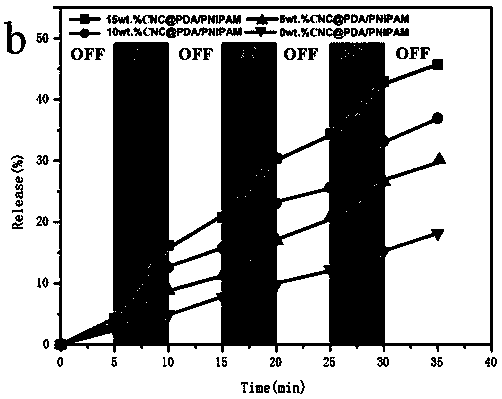
Cellulose/N-isopropylacrylamide medicine controllable release hydrogel and preparation method thereof
An isopropylacrylamide and hydrogel technology, which can be applied in pharmaceutical formulations, photosplitting of drugs in the body, and medical preparations with non-active ingredients, etc., to achieve improved drug loading capacity, excellent performance, and good biocompatibility sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) According to the mass ratio of cellulose raw material and sulfuric acid as 1:50, add cotton fiber raw material to 50wt% sulfuric acid solution, and obtain nanocellulose by ultrasonication for 3 hours at a temperature of 60°C, and dialyze the obtained nanocellulose After obtaining the nanocellulose solution;
[0028] (2) Dissolve 2.0 g of dopamine hydrochloride in 20 g of nanocellulose suspension with a mass fraction of 10.0% obtained in step (1), adjust the pH to 8.5 with hydrochloric acid, react at room temperature for 24 h, and modify the resulting After that, an appropriate amount of deionized water was added to the nanocellulose to prepare a nanocellulose solution with a mass fraction of 8.0%.
[0029] (3) Add 0.4g of N-isopropylacrylamide to 0.12g of the cellulose aqueous solution obtained in step (2), and then add 0.004g of N,N'-methylenebisacrylamide crosslinking agent, magnetic After stirring for 30-40 minutes, the near-infrared responsive cellulose / N-isoprop...
Embodiment 2
[0033] (1) According to the mass ratio of cellulose raw material and sulfuric acid as 1:50, the straw pulp fiber raw material was added to 50 wt % sulfuric acid solution, and nanocellulose was obtained by ultrasonication for 3 h at a temperature of 60 °C, and the obtained nanocellulose was Obtain nanocellulose solution after dialysis;
[0034] (2) Dissolve 2.0 g of dopamine hydrochloride in 20 g of nanocellulose suspension with a mass fraction of 10.0% obtained in step (1), adjust the pH to 8.5 with hydrochloric acid, react at room temperature for 24 h, and modify the resulting After that, an appropriate amount of deionized water was added to the nanocellulose to prepare a nanocellulose solution with a mass fraction of 8.0%.
[0035] (3) Add 0.4g of N-isopropylacrylamide to 0.4g of the cellulose solution obtained in step (2), and then add 0.004g of N,N'-methylenebisacrylamide crosslinking agent, Magnetically stirred for 30-40 minutes, the near-infrared responsive cellulose / N-...
Embodiment 3
[0039] (1) According to the mass ratio of cellulose raw material and sulfuric acid as 1:50, add microcrystalline cellulose raw material to 50 wt % sulfuric acid solution, and obtain nanocellulose by ultrasonication for 3 hours at a temperature of 60 °C, and the obtained nanofibers The nanocellulose solution is obtained after dialysis;
[0040] (2) Dissolve 2.0 g of dopamine hydrochloride in 20 g of nanocellulose suspension with a mass fraction of 10.0% obtained in step (1), adjust the pH to 8.5 with hydrochloric acid, react at room temperature for 24 h, and modify the resulting After that, an appropriate amount of deionized water was added to the nanocellulose to prepare a nanocellulose solution with a mass fraction of 8.0%.
[0041] (3) Add 0.4g of N-isopropylacrylamide to 0.8g of the cellulose solution obtained in step (2), then add 0.004g of N,N'-methylenebisacrylamide crosslinking agent, magnetic After stirring for 30-40 minutes, the near-infrared responsive cellulose / N-i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com