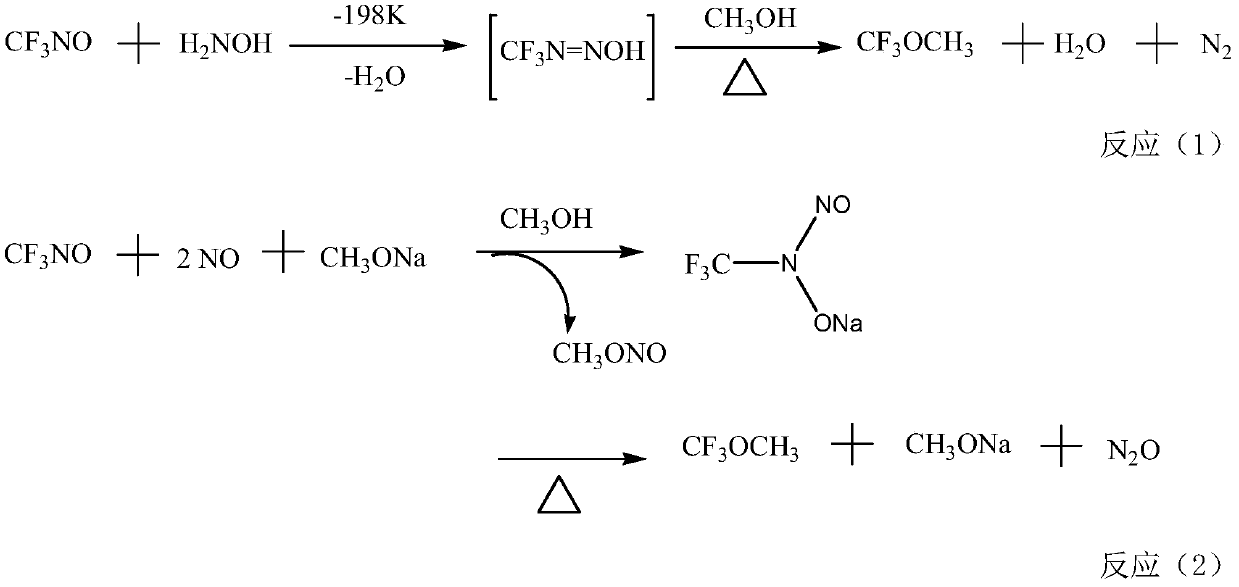
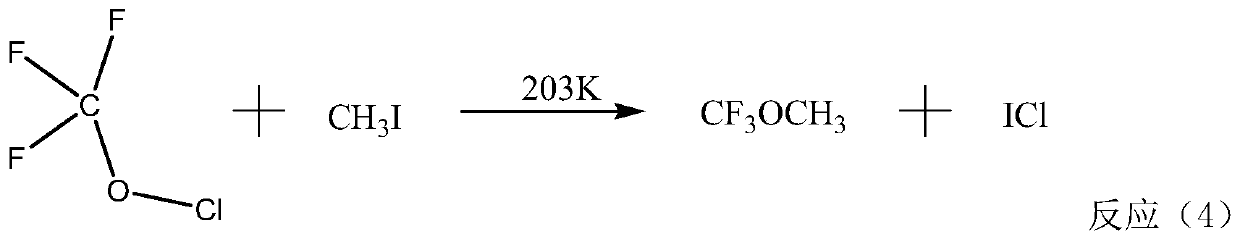
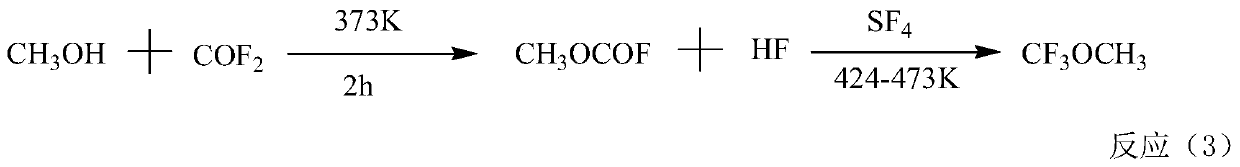Method for preparing fluorine-containing ether
A technology of fluorine-containing ethers and trifluoromethyl methyl ethers, which is applied in the field of preparation of fluorine-containing ethers, and can solve problems such as easy decomposition, high toxicity, and difficulty in obtaining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] In a 1L autoclave, add 200 ml of acetonitrile, 0.5 mol of CsF, and 2 mol of carbonyl fluoride, react at 20 degrees for 24 hours, then separate the remaining carbonyl fluoride in the above reaction system at -40 degrees, and then add 0.01mol of water and 1mol of dimethyl carbonate were reacted at 20 degrees for 24 hours. After the reaction, distillation was carried out to obtain trifluoromethyl methyl ether (boiling point was -25.2°C / 760mmHg), with a yield of 92.8% and a purity of was 98.1%.
Embodiment 2
[0083] In a 1L autoclave, add 200 ml of propionitrile, 0.5 mol of RbF, and 2 mol of trifluoroacetyl fluoride, react at 20 degrees for 24 hours, and then separate the remaining trifluoroacetyl fluoride in the above reaction system at -40 degrees , then add 0.03mol of water and 1mol of dimethyl carbonate, react at 20 degrees for 36 hours, after the reaction ends, carry out distillation to obtain pentafluoroethyl methyl ether (boiling point is 6 ℃ / 760mmHg), and the yield is 90.3% , with a purity of 97.2%.
Embodiment 3
[0085] In a 1L autoclave, add 200 ml of butyronitrile, 0.5 mol of KF, and 2 mol of pentafluoropropionyl fluoride, react at 20 degrees for 24 hours, and then separate the remaining pentafluoropropionyl in the above reaction system at -20 degrees. Acyl fluoride, then add 0.05mol of water and 1mol of dimethyl carbonate, and react at 20 degrees for 36 hours. After the reaction is finished, distill to obtain heptafluoropropyl methyl ether (boiling point is 6°C / 760mmHg), and the yield is 83.7% with a purity of 98.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com