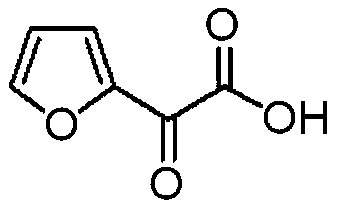
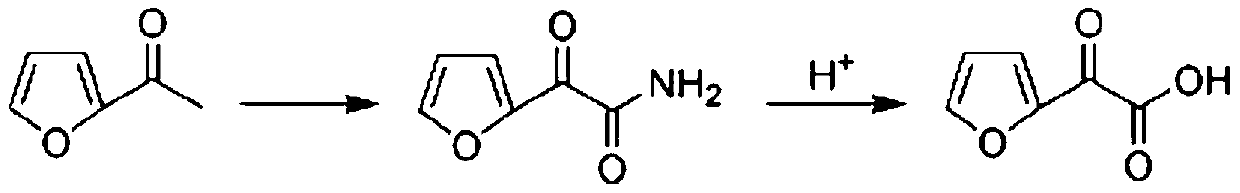
Preparation method of furanone acid
A technology of furanoic acid and acetylfuran, applied in the field of medicine and chemical industry, can solve the problems of many side reactions of furanoic acid, large amount of oxidant, low toxicity, etc., and achieves the advantages of reducing side reactions, reducing the generation of by-products, and improving yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Preparation of nitrite
[0038] Add 120mL of water, 40g of n-butanol, and 31.8g of concentrated sulfuric acid into a 500mL reaction bottle, and cool it to 0°C in an ice bath, and slowly add 112g of 40% sodium nitrite aqueous solution to it for 1.5 hours, and the temperature during the dropping process should not exceed 5 °C, the dropwise addition was completed, stirred for 30 min, and the ester layer was separated to obtain 51 g of butyl nitrite.
[0039] (2) Preparation of furanone acid
[0040] Dissolve 20g of 2-acetylfuran in 150mL of 10% dilute hydrochloric acid solution, add 2g of concentrated sulfuric acid, 0.1g of catalyst γ-Al 2 o 3 / Pd(II), control the temperature to 40-55°C, add 20.6g of n-butyl nitrite dropwise for 1h, continue to stir for 1h after the dropwise addition, adjust the pH to 2.8 after the reaction, filter and recover the catalyst, the filtrate Add 100 mL of dichloromethane to extract n-butanol and unreacted acetylfuran produced by the reac...
Embodiment 2
[0043] The implementation conditions are the same as in Example 1. After the furanone acid aqueous solution is prepared, add 110 g of 10% methoxyamine aqueous solution to the furanone acid aqueous solution for oximation reaction for 4 hours, then add 60 g of industrial salt, acidify to pH<0.5, and use 100 mL×3 extracted three times with dichloromethane, combined the organic phases and added methanolic ammonia dropwise to pH 7.0, filtered to obtain 23 g of ammonium furan salt, and the molar yield was 68% based on acetylfuran.
Embodiment 3
[0045](1) Preparation of nitrite
[0046] Add 120mL of water, 30g of n-hexanol, and 16g of concentrated sulfuric acid into a 500mL reaction bottle, and cool it to 0°C in an ice bath, and slowly add 55.7g of 40% sodium nitrite aqueous solution to it for 1.5 hours, and the temperature during the dropping process should not exceed 5°C , the dropwise addition was completed, stirred for 30 min, and the ester layer was separated to obtain 37 g of hexyl nitrite.
[0047] (2) Preparation of furanone acid
[0048] Dissolve 20g of 2-acetylfuran in 150mL of 10% dilute hydrochloric acid solution, add 2g of concentrated sulfuric acid, 0.1g of catalyst γ-Al 2 o 3 / Pd(II), control the temperature to 45-50°C, add 25g of hexyl nitrite dropwise for 1h, continue to stir for 1h after the dropwise addition, adjust the pH to 3.0 after the reaction, filter and recover the catalyst, add 100mL of The n-hexanol produced by dichloromethane extraction reaction and the unreacted acetylfuran, the water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com