Macrocyclic polyamine amphiphile compound based on green fluorescent protein chromophore bi and its preparation method and use
A green fluorescent protein and macrocyclic polyamine technology, which is applied in the field of macrocyclic polyamine amphiphiles and their preparation, can solve the problems of low transfection efficiency, single performance, and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A kind of macrocyclic polyamine amphiphile compound based on green fluorescent protein chromophore B1, the structural formula (I) of said compound is as follows:
[0047]
[0048] In formula (I), R is a hydrocarbyl structural unit.
[0049] Wherein, when R is a linear alkyl structural unit, and R is , the synthesized macrocyclic polyamine amphiphile based on the green fluorescent protein chromophore BI is denoted as BI-A; R is The synthesized macrocyclic polyamine amphiphile based on the green fluorescent protein chromophore BI is denoted as BI-B; R is The synthesized macrocyclic polyamine amphiphile based on the green fluorescent protein chromophore BI is denoted as BI-C; R is The synthesized macrocyclic polyamine amphiphile based on the green fluorescent protein chromophore BI is denoted as BI-D. Concrete synthetic route is as follows:
[0050]
[0051] In the formula, (i) bromoalkane RBr, potassium carbonate, acetone, reflux, 48 hours; (ii) 3,5-bis (azi...
Embodiment 2
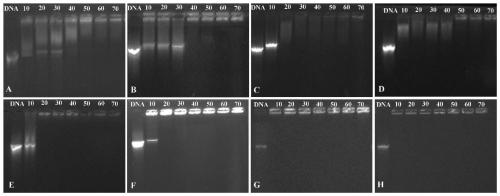
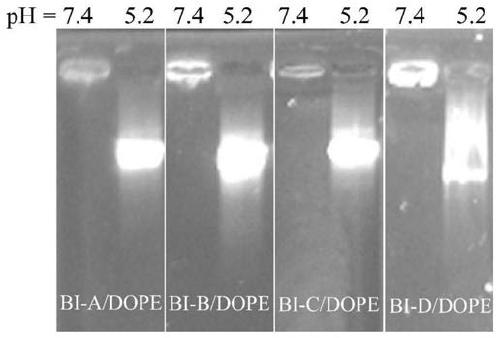
[0070] Prepare solutions of different concentrations of compound BI-A~BI-D respectively, place different concentrations of BI-A~BI-D and pUC18 plasmid DNA (9μg / mL) in a 37°C water bath, incubate for 1h, and then carry out DNA agarose Gel retardation experiment The gel retardation results of different concentrations of compounds on pUC18 DNA were obtained.
[0071] figure 1 A~1H are the results of agarose gel retardation experiments on pUC18 DNA by compounds BI-A~BI-D and BI-A / DOPE~BI-D / DOPE (the ratio of compound to DOPE is 1:3) of the present invention, respectively; figure 1 The value marked above in A~1H is the test concentration (μM); figure 1 A~1H shows that compounds BI-A~BI-D and BI-A / DOPE~BI-D / DOPE (the ratio of compound to DOPE is 1︰3) can completely block the migration of DNA in agarose at lower concentrations .
[0072] It can be concluded from Example 2 that the amphiphilic compound based on the BI unit prepared in the present invention can effectively condense ...
Embodiment 3
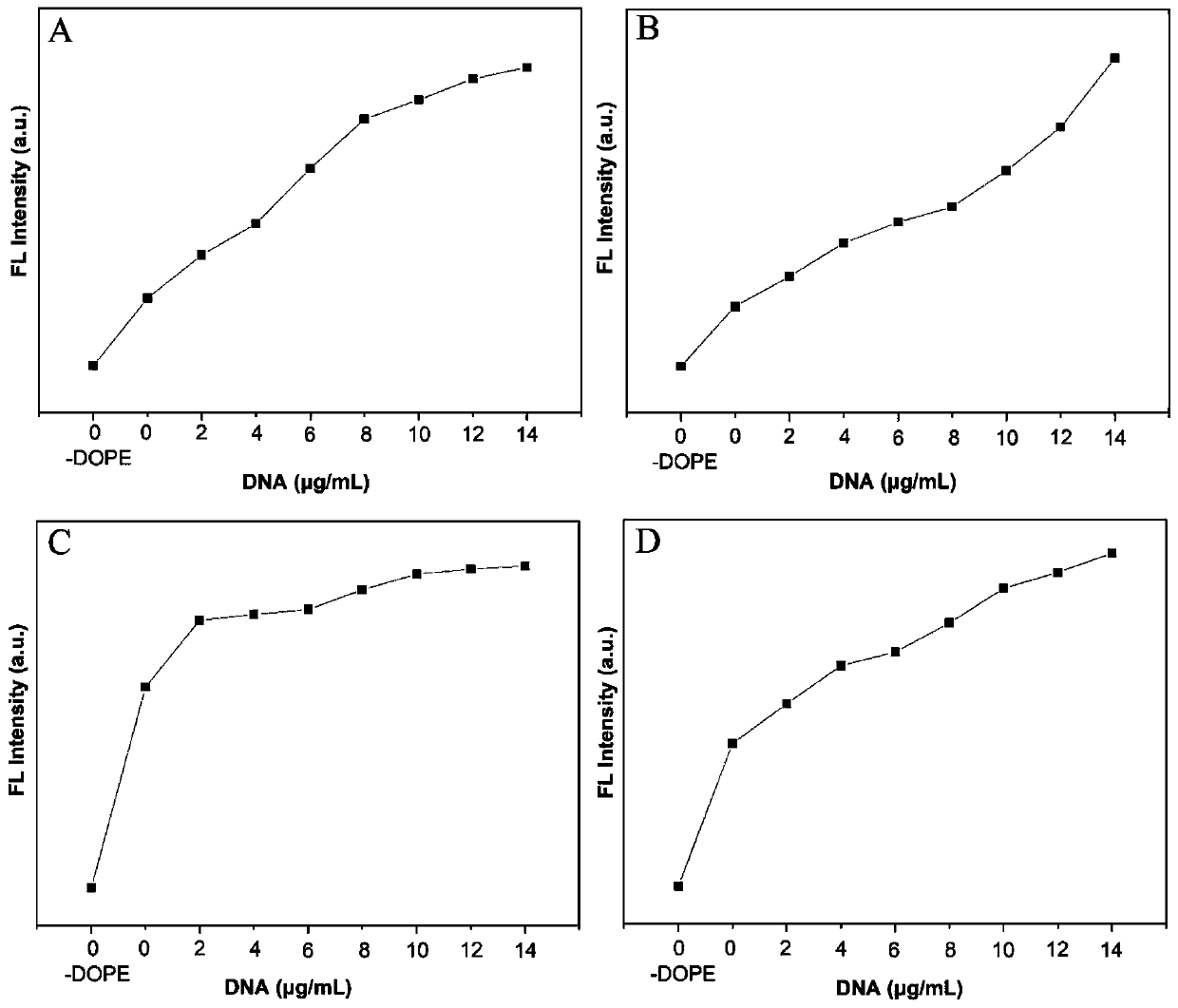
[0074] Add ctDNA to the solution of cationic liposome BI-A / DOPE, BI-B / DOPE, BI-C / DOPE and BI-D / DOPE (the ratio of compound to DOPE is 1:3), test its fluorescence intensity and drawing, get figure 2 A~2D.
[0075] figure 2 A~2D are the fluorescence titration results of ctDNA to the complexes BI-A / DOPE, BI-B / DOPE, BI-C / DOPE and BI-D / DOPE (the ratio of compound to DOPE is 1︰3); among them, the X axis is the concentration of DNA, and the Y axis represents the fluorescence intensity. It can be concluded from Example 3 that the present invention artificially simulates the chromogenic process of green fluorescent protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com