Environment-friendly method for preparing antibacterial medicine cefodizime acid with low cost
A technology for ceftazidime and antibacterial drugs, which is applied in the new preparation field, can solve the problems of low yield, poor reaction degree and high impurities, and achieves the effects of reducing cost, saving energy consumption and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
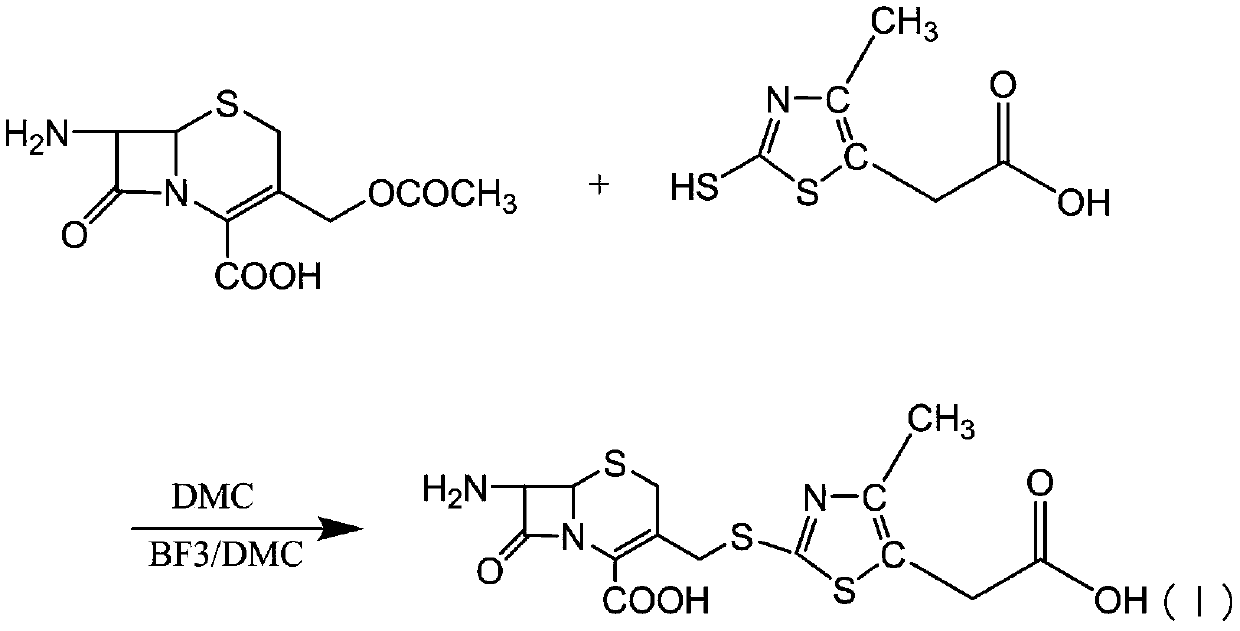
[0044] At room temperature, 150 ml of dimethyl carbonate, 20 g of 7-aminocephalosporanic acid, and 15 g of MMTA were added. Add 15g of boron trifluoride-dimethyl carbonate complex and 2g of methanesulfonic acid at the same temperature, control the temperature at 35-40°C and time the reaction for 1-2.0hr, then lower the temperature to below 20°C and add 320ml of pure water for hydrolysis.
[0045] Then add 2g of activated carbon for decolorization and filter for 20min. Control the temperature of the filtrate below 5°C, add dropwise a dilute alkali solution to adjust the pH to 3.8-4.0, and precipitate crystals. After filtering, the filter cake was washed with pure water, and then washed with acetone after draining, and the wet product of TACS was obtained after draining.
[0046] The purity of the high-pressure liquid phase is 98.2%, and the color grade is lower than the yellow-green standard colorimetric solution No. 3.
Embodiment 1-2
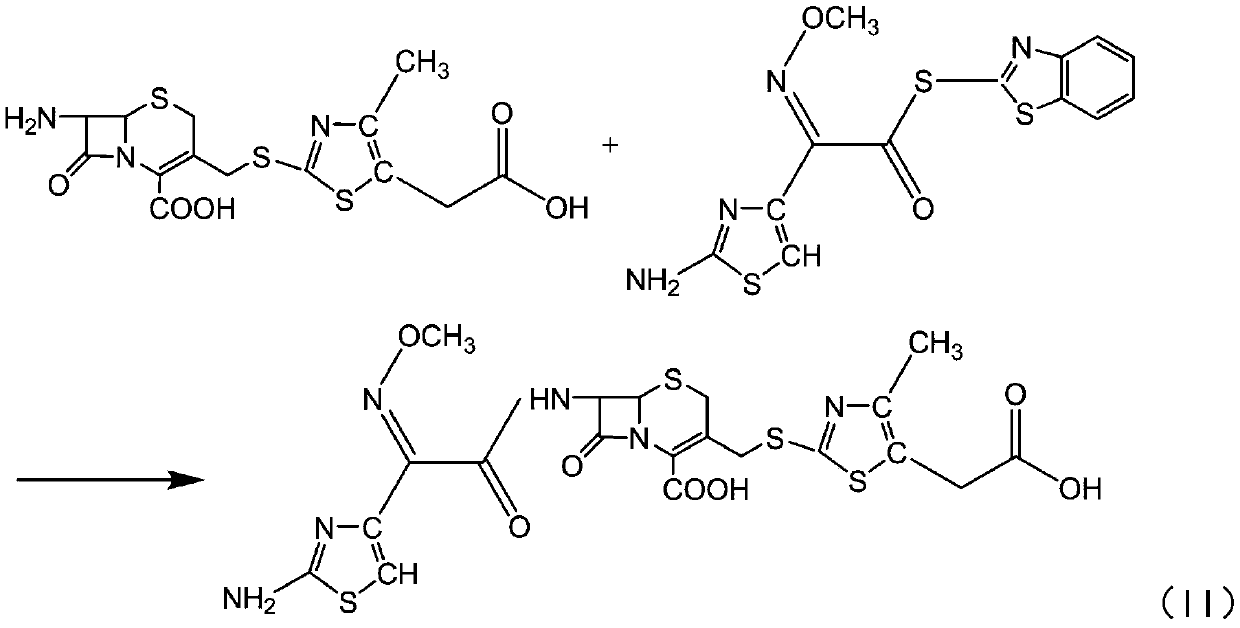
[0048] Add 150ml of dichloromethane to the reactor to control the temperature below 10 degrees, add 24ml of methanol, add the above TACS wet product, add 2ml of triethylamine dropwise under stirring to dissolve it, and then add 26g of AE-active ester. Timed reaction 2.0-5.0hr. Add 500ml of pure water to extract the organic phase, then add 2g of activated carbon to the water phase to decolorize for 20min, filter, wash the carbon cake with 30ml of water, add ethanol to the filtrate, add dilute acid to acetone to adjust the pH=3.5-3.7 to precipitate crystals. Suction filtration, wash the filter cake with pure water, and drain. The filter cake was washed with acetone and drained. The dried cefodizime acid is 28.5g.
[0049] The purity of the high-pressure liquid phase is 98.9%, and the color grade is lower than the yellow-green standard colorimetric solution No. 3.
Embodiment 2-1
[0051] At room temperature, 120ml of dimethyl carbonate, 20g of 7-aminocephalosporanic acid, and 15g of MMTA were added. Add 10 g of boron trifluoride-dimethyl carbonate complex and 5 days of concentrated sulfuric acid at the same temperature, control the temperature at 35-40°C and time the reaction for 1-2.0 hours, then cool down to below 20°C and add 320ml of pure water for hydrolysis. Then add 2g of activated carbon for decolorization and filter for 20min. Control the temperature of the filtrate below 5°C, add dropwise a dilute alkali solution to adjust the pH to 3.8-4.0, and precipitate crystals. After filtering, the filter cake was washed with pure water, and then washed with acetone after draining, and the wet product of TACS was obtained after draining.
[0052] The purity of the high-pressure liquid phase is 98.5%, and the color grade is lower than the yellow-green standard colorimetric solution No. 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com