Organic light-emitting materials containing dinitrogen difluoride-boron-oxygen heterocyclic receptor structural unit and application thereof
A luminescent material, a technology of nitrogen difluoride, which is applied in the field of organic luminescent materials, OLED display and lighting, and can solve the problems that blue phosphorescent materials are difficult to meet the requirements of commercial applications, limited metal phosphorescent materials, and limited resources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
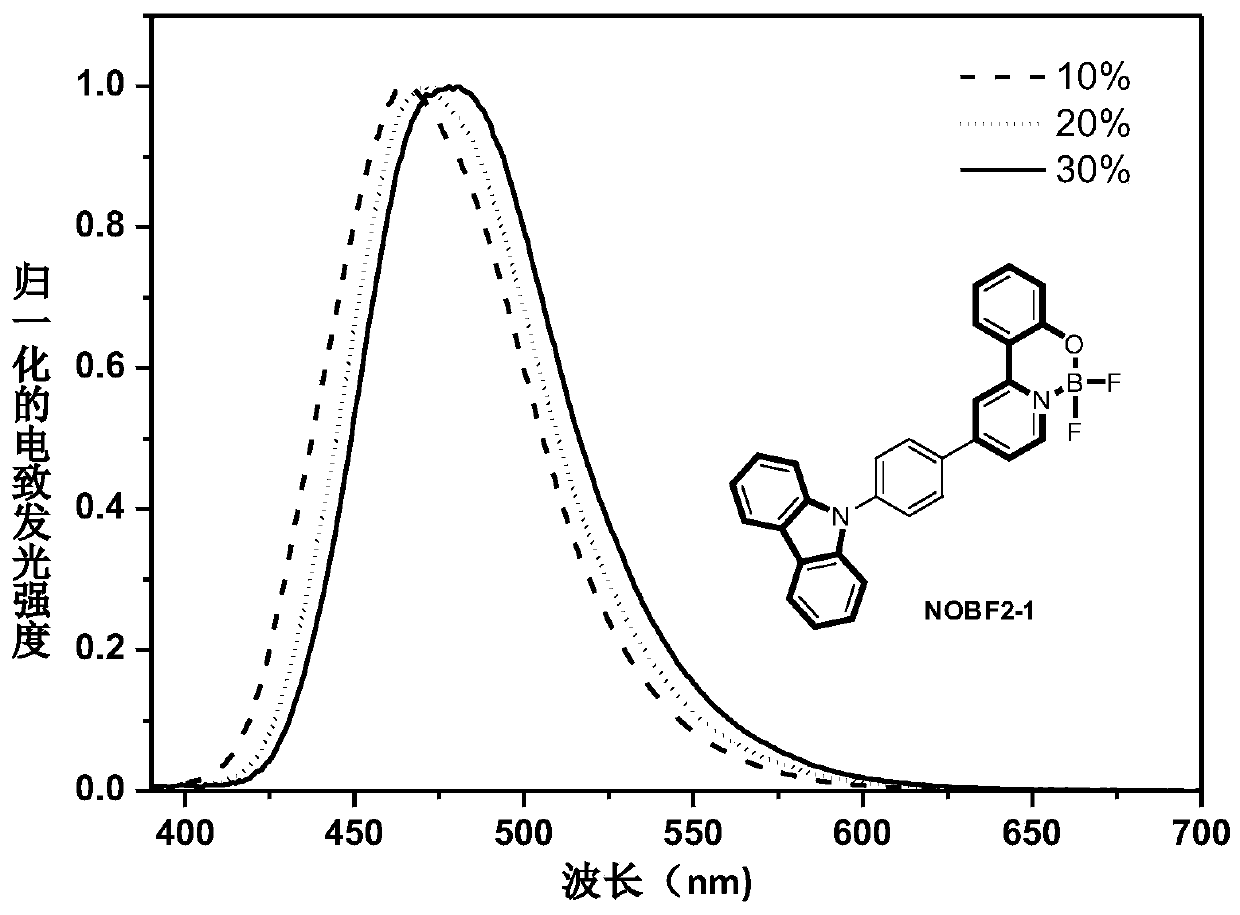
[0081] Embodiment 1: The luminescent material NOBF2-1 can be synthesized according to the following route:
[0082]
[0083] Synthesis of Intermediate 1: Add 2,4-dibromopyridine (15.72g, 66.40mmol, 1.0 equivalent), 2-methoxyphenylboronic acid (11.10g ,73.01mmol,1.1eq), Pd(PPh 3 ) 4 (2.30g, 1.99mmol, 3mol%), Na 2 CO 3 (21.11g, 199.20mmol, 3.0 equivalents), nitrogen was pumped three times. Tetrahydrofuran (120 mL) and water (30 mL) were added under nitrogen protection. The mixture was stirred and reacted in an oil bath at 85°C for 48 hours, monitored by thin-layer chromatography until the reaction of the raw materials was completed, and then cooled to room temperature. The reaction mixture was extracted with ethyl acetate (50mL×3), the combined organic phases were washed with anhydrous Na 2 SO 4 dry. After filtration, the solvent was distilled off under reduced pressure, and the crude product was separated and purified by silica gel chromatography. The eluent was petr...
Embodiment 2
[0089] Embodiment 2: The luminescent material NOBF2-2 can be synthesized according to the following route:
[0090]
[0091] Synthesis of intermediate OCH3-2: Add intermediate 2 ((2.49g, 8.41mmol, 1.0 equivalent), 3,6-di-tert-butylcarbazole ( 2.59g, 9.25mmol, 1.1 equiv), Pd 2 (dba) 3 (231.0 mg, 0.25 mmol, 3.0 mol%), sodium tert-butoxide (1.62 g, 16.82 mmol, 2.0 equiv). The nitrogen was replaced three times, and tri-tert-butylphosphine (1.6mL, 0.67mmol, 8.0mol%; 10wt% hexane solution, density: 0.861 g / mL) and o-xylene 30mL were added under the protection of nitrogen, and the temperature of the oil bath was raised to Stir and react at 130°C for 12.0 hours, monitor by thin-layer chromatography until the reaction of the raw materials is complete, and cool to room temperature. The solvent was distilled off under reduced pressure, the sample was loaded by dry method, separated and purified by silica gel column chromatography, and the eluent was petroleum ether / ethyl acetate =...
Embodiment 3
[0095] Embodiment 3: The luminescent material NOBF2-3 can be synthesized according to the following route:
[0096]
[0097] Synthesis of intermediate OCH3-3: Intermediate 2 (592 mg, 2 mmol, 1.0 equivalent), 3,6-diphenylcarbazole (2.4 mmol, 767 mg, 1.2 equivalent), tert-butyl Sodium alkoxide (6mmol, 577mg, 3.0eq) and Pd(OAc) 2 (0.06mmol, 14mg, 3mol%). Then replace nitrogen three times, inject t-Bu 3 P (376μl, 0.16mmol, 8mol%, 10wt% hexane solution, density; 0.861g / mL), xylene (36mL), refluxed in an oil bath at 150°C for 48 hours, and monitored by thin-layer chromatography until the reaction of the raw materials was complete. Add a small amount of water to quench, extract three times with ethyl acetate, combine the organic phases, and use Na 2 SO 4 Dry, filter, and distill off the solvent under reduced pressure. The crude product was separated and purified by silica gel column chromatography, eluent: petroleum ether / ethyl acetate=10:1-3:1, and 1149 mg of a light yellow s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com