Phenylacetyltetrahydro-beta-carboline small molecule compound and application thereof
A technology based on phenylacetyl and carbolines, which is applied in the field of biomedical chemistry and can solve problems such as loss of efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the preparation of WG-449E
[0051] Place methyl indole-7-carboxylate in a round-bottomed flask, dissolve it with TFA, stir and react for 10 minutes, add the DCM solution in which 1-dimethylamino-2-nitroethylene is dissolved into the flask dropwise, and then React at room temperature for about 2 hours. TLC detection, after the completion of the reaction, the reaction solution was evaporated to the minimum amount, with a little excess saturated NaHCO 3 Neutralize the concentrated solution until the pH is about 8. At this time, a large number of yellow solids are precipitated in the solution, filtered by suction, washed repeatedly, dried and ground to obtain a yellow powder;
[0052] The obtained yellow powder was dissolved in methanol solution, and NaBH was added in batches under ice-cooling conditions. 4 , N 2 protection, react for about 5 hours until the solution becomes colorless; add an appropriate amount of palladium carbon powder (10%), stir for 5 ...
Embodiment 2
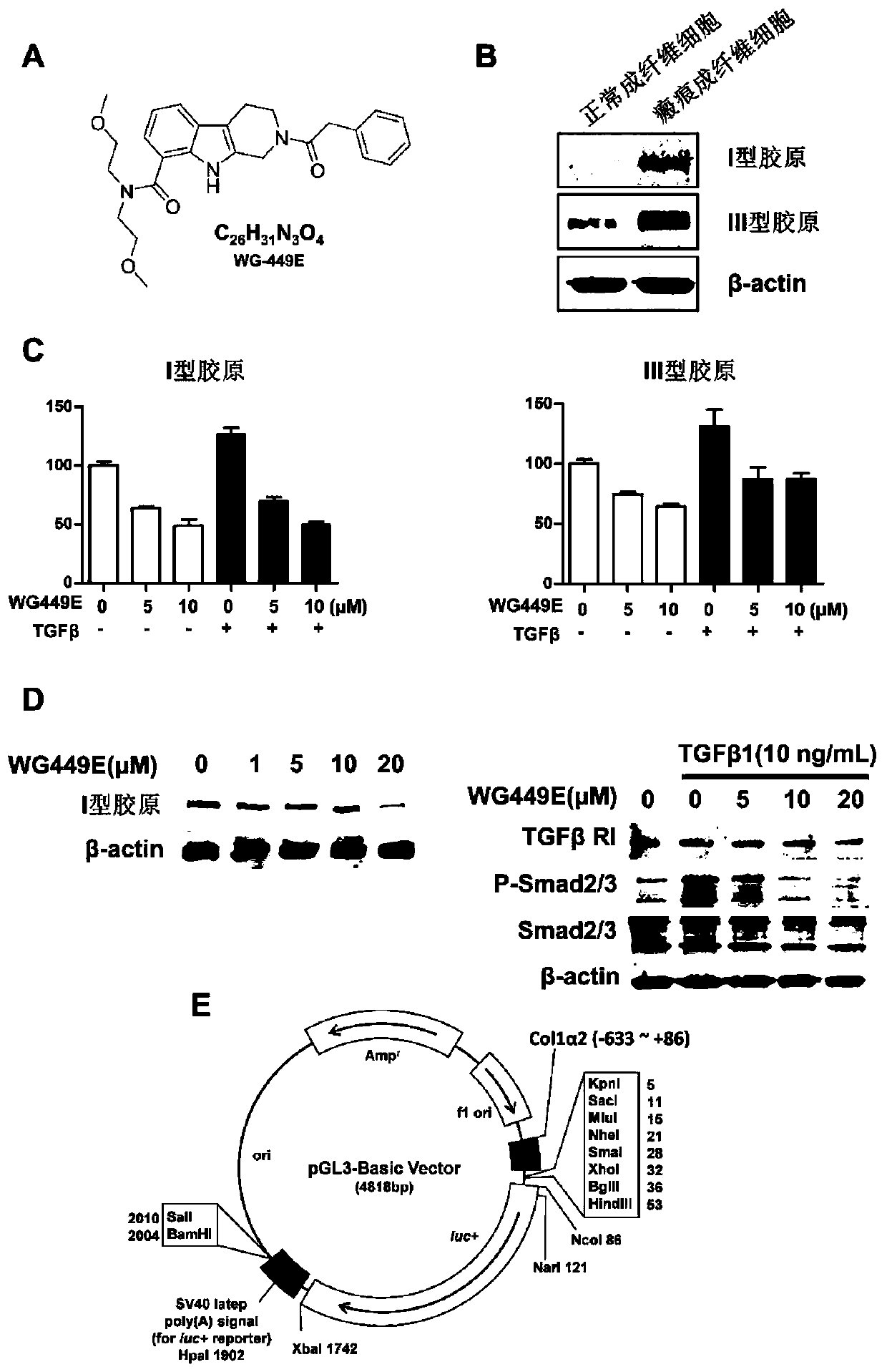
[0059] Example 2: WG-449E inhibits gene transcription and protein expression of type I collagen.
[0060] 1. The effect of WG-449E on gene transcription of type I collagen detected by luciferase double reporter gene
[0061]Purpose and principle: Bioluminescent organisms are widely distributed in nature, and the enzymes that catalyze these bioluminescent reactions are called luciferases. Under suitable substrate conditions, luciferase can quickly catalyze the oxidation of the luciferin substrate, and the signal generated during the oxidation process can be captured by a fluorescence detector. The strength of the signal was used to judge the luciferase activity of the sample. Since endogenous luciferase is not distributed in mammalian cells, luciferase is an ideal reporter gene. By cloning the target gene upstream of the luciferase gene, constructing a reporter gene plasmid, transfecting cells, lysing the cells, and measuring the activity of luciferase, the effect of drug add...
Embodiment 3
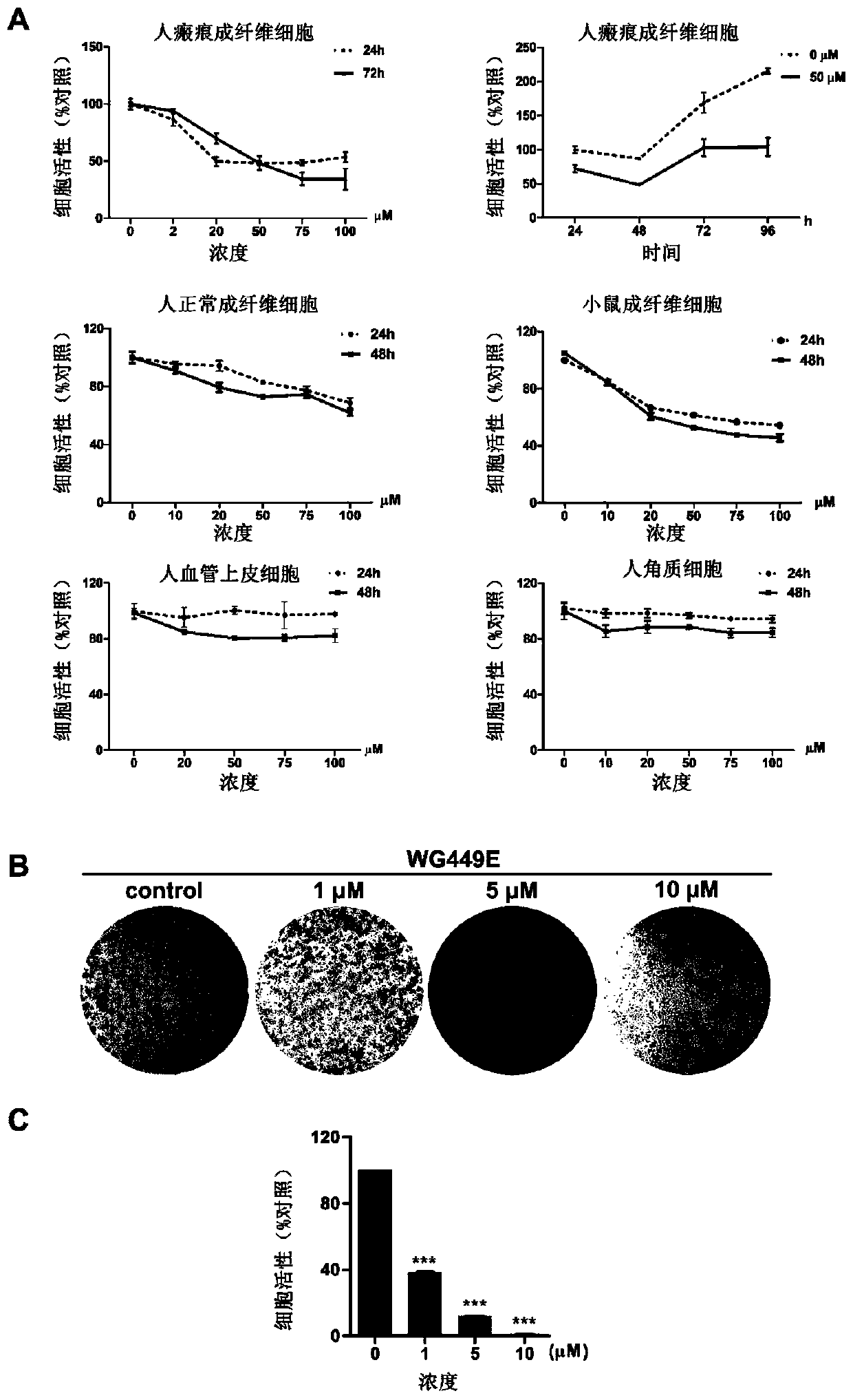
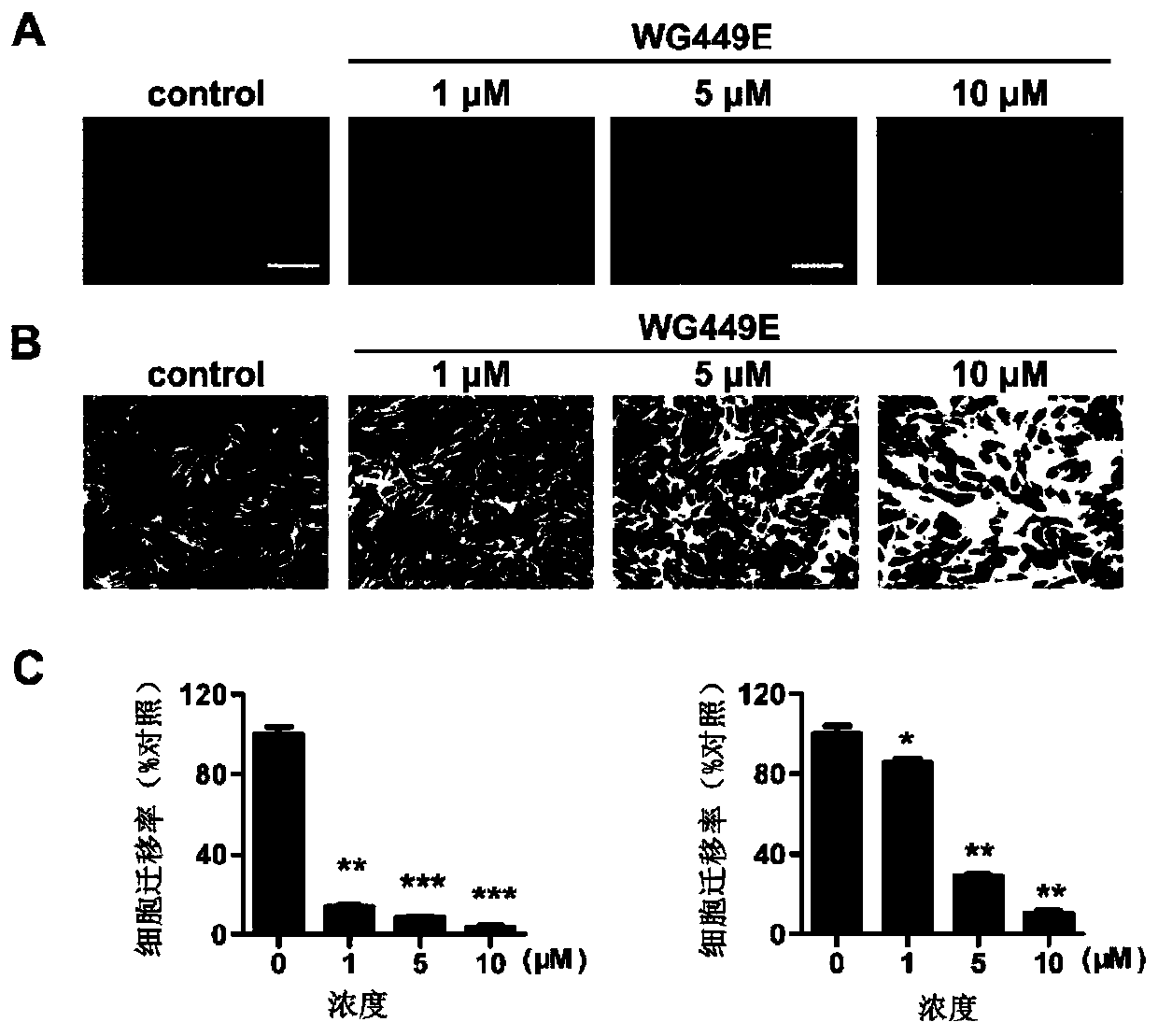
[0067] Example 3: WG-449E inhibits the proliferation and migration of scar fibroblasts at a non-toxic dose.
[0068] 1. SRB method was used to detect the toxicity of WG-449E of the present invention to normal skin cells and scar fibroblasts.
[0069] Purpose and principle: The absorption peak of sulforhodamine B (SRB) at 540nm wavelength has a linear relationship with the cell mass, so it is often used as a quantitative detection method for cell proliferation ability.
[0070] Method: Add scar fibroblasts into 96-well plate, after the cells adhere to the wall, replace with fresh medium with different concentrations of WG-449E, after 24, 48, 72, and 96 hours of drug treatment, fix the cells, wash them, and air-dry them Add SRB dye solution for staining for 10min, wash off excess dye solution with 1% acetic acid, and air dry. The Tris solution dissolves the SRB bound to the cell protein, and after shaking, the absorbance value of each well at a wavelength of 515nm is measured w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com