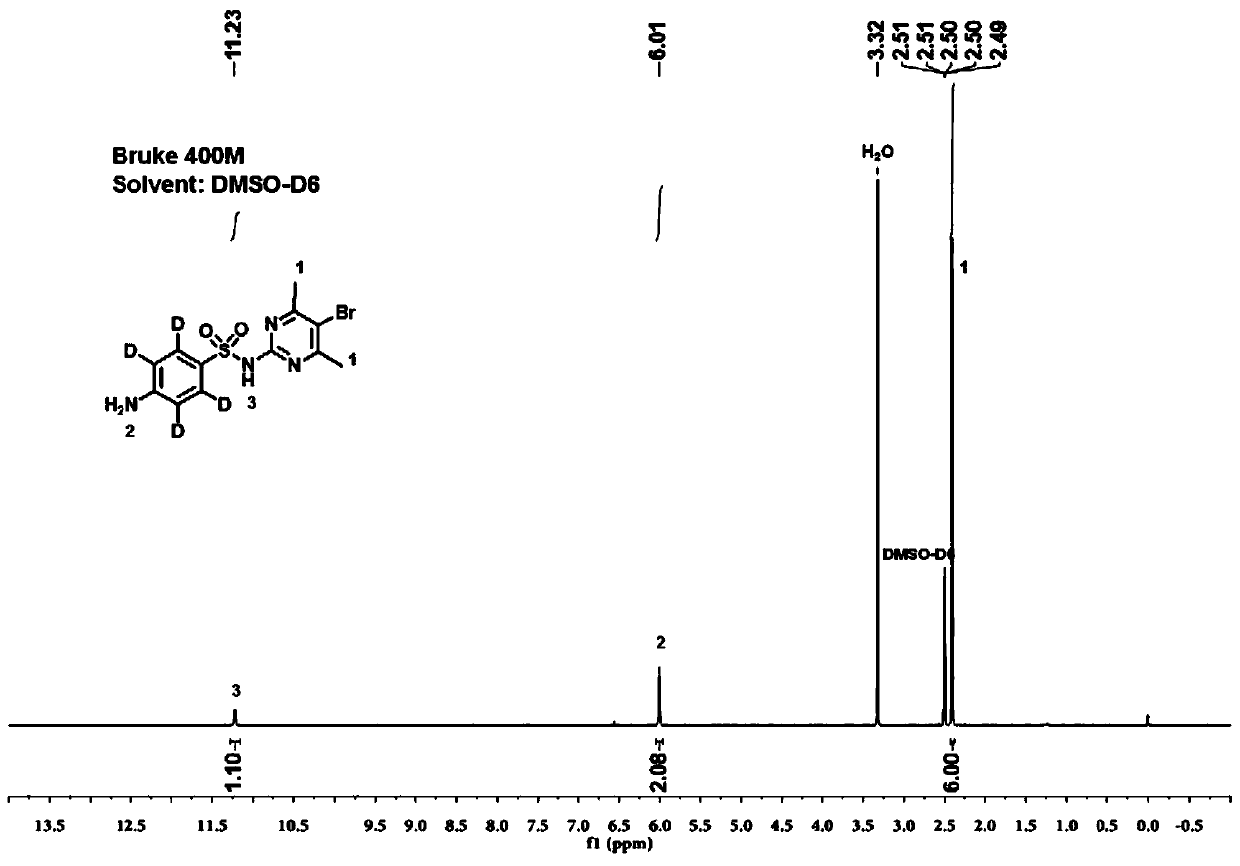
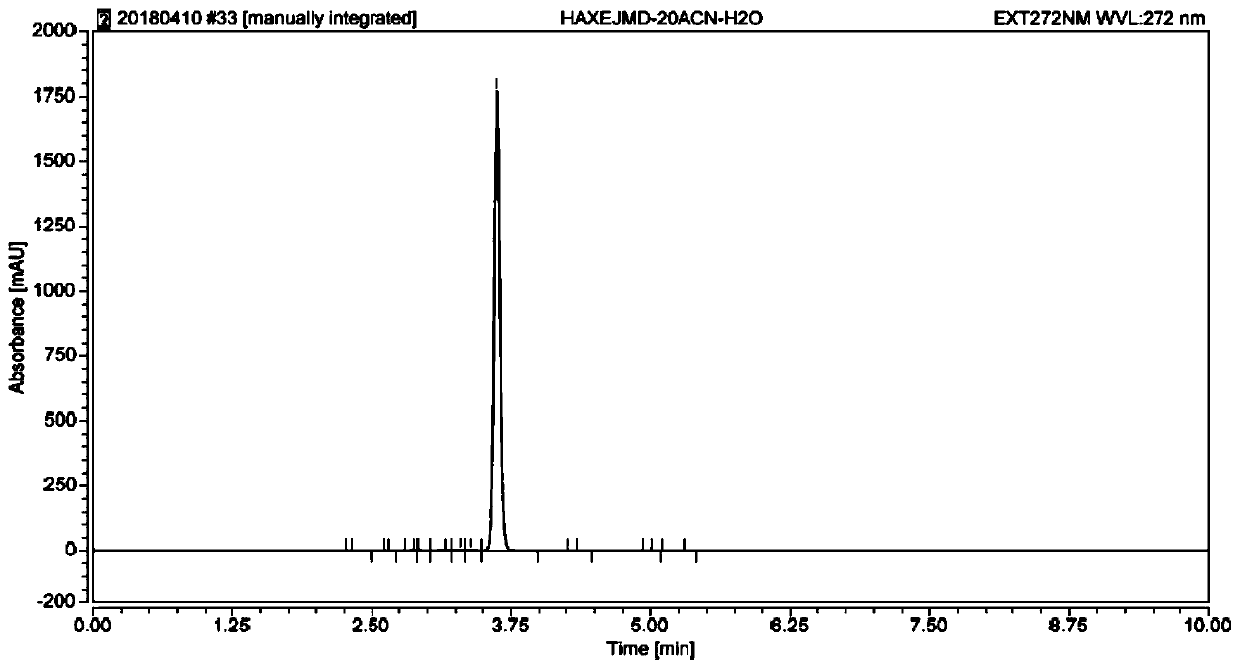
Stable isotope labelled sulfamethazine and synthesis method thereof
A technology of sulfabromomethazine and stable isotopes, which is applied in the field of stable isotope-labeled sulfabromethazine and its synthesis, can solve the problems of long synthesis process route, expensive raw materials, difficult production process, etc., and achieve short process route , good economy and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthetic process of stable isotope-labeled sulfabromomethazine is as follows:
[0039] S1. Under ice-salt bath and nitrogen protection, add aniline-D to the reaction vessel 5 (17.96mmol, 1.76g), acetic anhydride (39.56mmol, 3.72mL) was added dropwise at 1 drop / second, reacted at 25-30°C for 2.5 hours, neutralized with saturated sodium bicarbonate solution, extracted with dichloromethane, and the organic phase was saturated Washing with brine, and removing the solvent from the organic phase to obtain acetaminophen-D 5 , yield 99%;
[0040] S2. Under ice-salt bath and nitrogen protection, add acetaminophen-D to the reaction vessel 5 (17.16mmol, 2.41g), chlorosulfonic acid (184mmol, 12.3mL) was added dropwise at 2 drops / second, reacted at 25-30°C for 3 hours, placed again in an ice-salt bath, and added dropwise at 2 drops / second on ice Water (278mmol, 5mL), the precipitated solid was filtered, the filter cake was washed with water, and the filter cake was dried at ...
Embodiment 2
[0045] The synthetic process of stable isotope-labeled sulfabromomethazine is as follows:
[0046] S1. Under ice-salt bath and nitrogen protection, add aniline-D to the reaction vessel 5 (15mmol, 1.5g), acetic anhydride (30mmol, 2.8mL) was added dropwise at 2 drops / second, reacted at 25-30°C for 4 hours, neutralized with saturated sodium bicarbonate solution, extracted with dichloromethane, organic phase saturated saline After washing, the organic phase removes the solvent to obtain acetaminophen-D 5 , yield 98%;
[0047] S2. Under ice-salt bath and nitrogen protection, add acetaminophen-D to the reaction vessel 5 (12mmol, 1.7g), chlorosulfonic acid (120mmol, 8mL) was added dropwise at 1 drop / second, reacted at 25-30°C for 4 hours, placed under ice-salt bath again, and ice water was added dropwise at 2 drops / second ( 250mmol, 4.5mL), precipitated solid, filtered, washed the filter cake with water, and dried the filter cake at 70-80°C for 2-3 hours to obtain p-acetamidobenze...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com