Reporter gene assay for measuring biological activity of recombinant human keratinocyte growth factors
A biological activity and reporter gene technology, applied in the field of recombinant drug activity detection, can solve the problems of high initial drug concentration, large result variability, long test period, etc., and achieve the effects of simple operation, high accuracy and small variation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
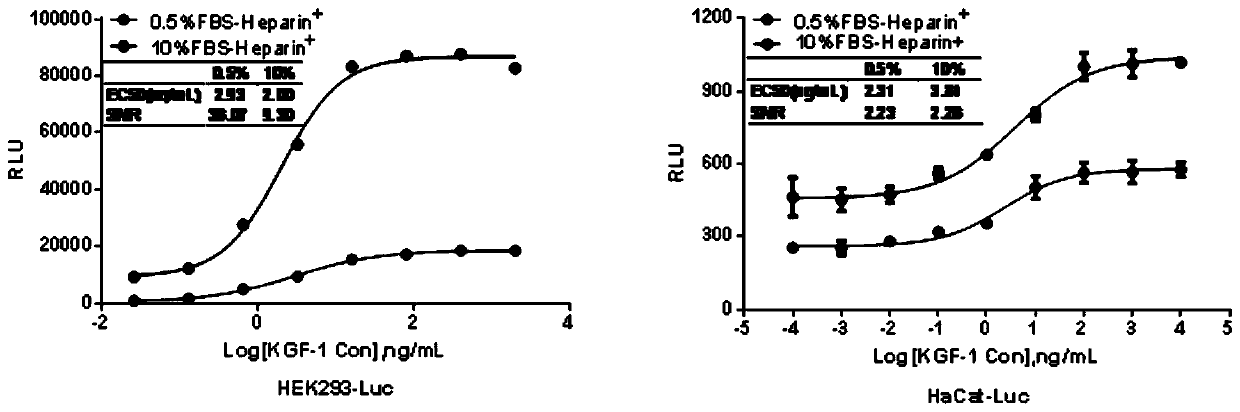
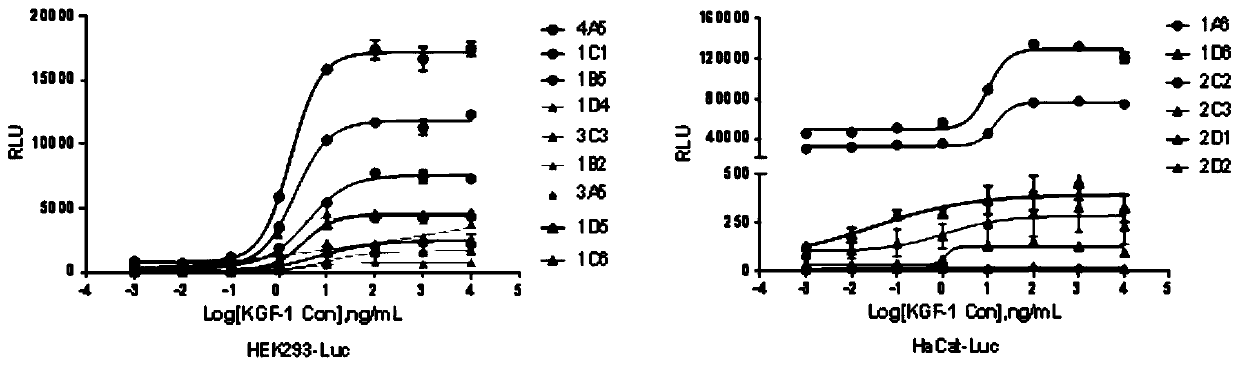
[0038] Example 1 Screening HEK293 and HaCat stable cell lines expressing SRE and KGFR2IIIb receptors
[0039] 1. Materials and methods
[0040] 1.1 cells
[0041] HEK293 cells were derived from ATCC; HaCat cells were derived from the Cell Bank of the Chinese Academy of Sciences.
[0042] 1.2 Reagents and materials
[0043] pGL4.33[luc2p / SRE / Hygro] plasmid and ViaFect TM Transfection reagents were purchased from Promega; KGFR2IIIb gene overexpression lentivirus was constructed and packaged by Shanghai Jikai Gene Technology Co., Ltd.; DMEM and fetal bovine serum (FBS) were purchased from GIBCO; puromycin was purchased from Invitrogen; hygromycin B was purchased from Suolaibao Biotechnology Co., Ltd.; Britelite plus luciferase substrate was purchased from PerkinElmer; white bottom light-transmitting 96-well plate was purchased from CORNING; recombinant KGF-1 and KGF-2 were retained by the Recombinant Drug Department of China National Institutes for Food and Drug Control.
[004...
Embodiment 2K
[0062] The methodological optimization of embodiment 2 KGF activity assay
[0063] 1. KGF-1 concentration optimization
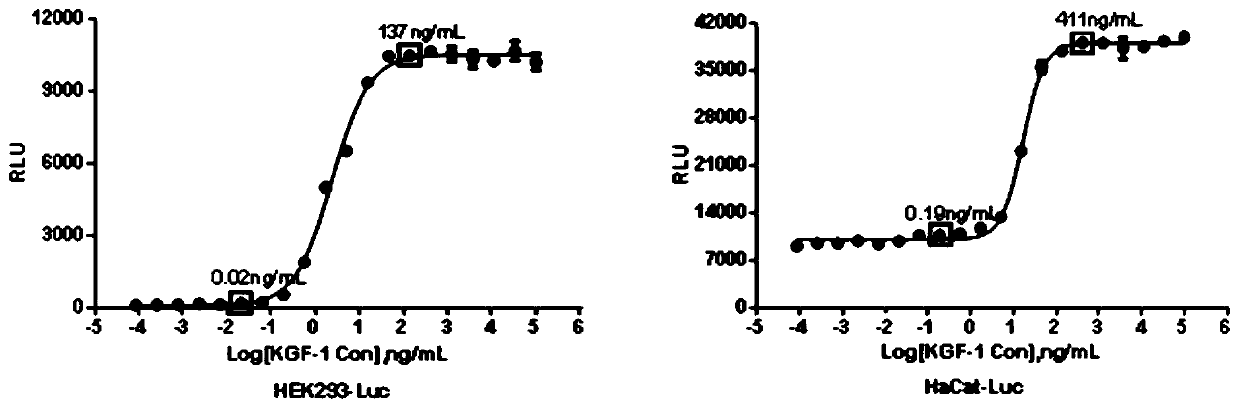
[0064] According to the monoclonal cell strain screening conditions in 2.5 of Example 1, the KGF-1 pre-diluted concentration was 100,000 ng / ml, and 20 gradients were serially diluted by 3 times, and 2 duplicate holes were set for each. Add equal volumes of different concentrations of recombinant KGF-1, act for 4-6h to detect the fluorescence signal value and fit the four-parameter curve ( image 3 ).
[0065] The results show that the pre-diluted concentrations of HEK293-Luc and HaCat-Luc cell lines are about 137ng / mL and 411ng / mL, and the 3-fold serial dilution of 8 concentrations is on the lower plateau of the curve. Therefore, choose 150ng / mL and 400ng / mL The concentration of recombinant KGF-1 was used as the pre-diluted concentration of HEK293-Luc and HaCat-Luc cell lines, and was diluted 3 times.
Embodiment 3K
[0090] The methodological verification of embodiment 3KGF activity assay
[0091] 1. Precision
[0092] The precision of the method was evaluated using the experimental conditions determined in Example 2. One batch of recombinant KGF-1 samples was taken for activity determination, which was measured 3 times a day for 4 consecutive days, with 3 replicate wells for each dilution.
[0093] The results in Table 7 show that the intraday coefficient of variation (CV) of the HEK293-Luc cell line is 1.49-3.75%, and the intraday CV value is 2.82% after continuous measurement for 4 days; the intraday coefficient of variation (CV) of the HaCat-Luc cell line is 0.52-5.78 %, the intraday CV value is 5.57%. The intraday and interday CV values of the two cell lines are both less than 6%, which shows that the precision of the method is good and can be used to measure the biological activity of recombinant KGF-1.
[0094] Table 7 The precision of HEK293-Luc and HaCat-Luc cell lines to mea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com