A kind of preparation method of pyridopyrimidine derivative
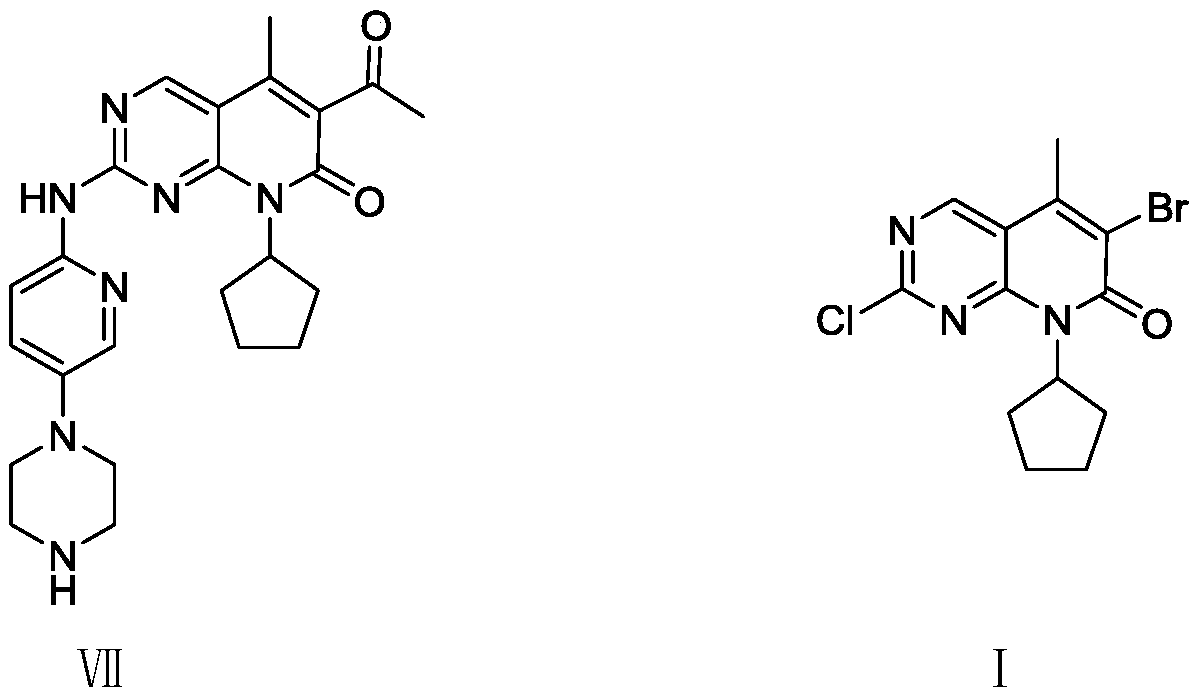
A technology for pyrimidine and cyclopentyl pyridine, which is applied in the field of preparation of pyridopyrimidine derivatives, a key intermediate of palbociclib, can solve the problem of unfavorable green industrial production of intermediate I, cost reduction, and low reaction atom economy. , unstable yield and other problems, to avoid heavy metal residues in the product, high reaction atom economy, and stable yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
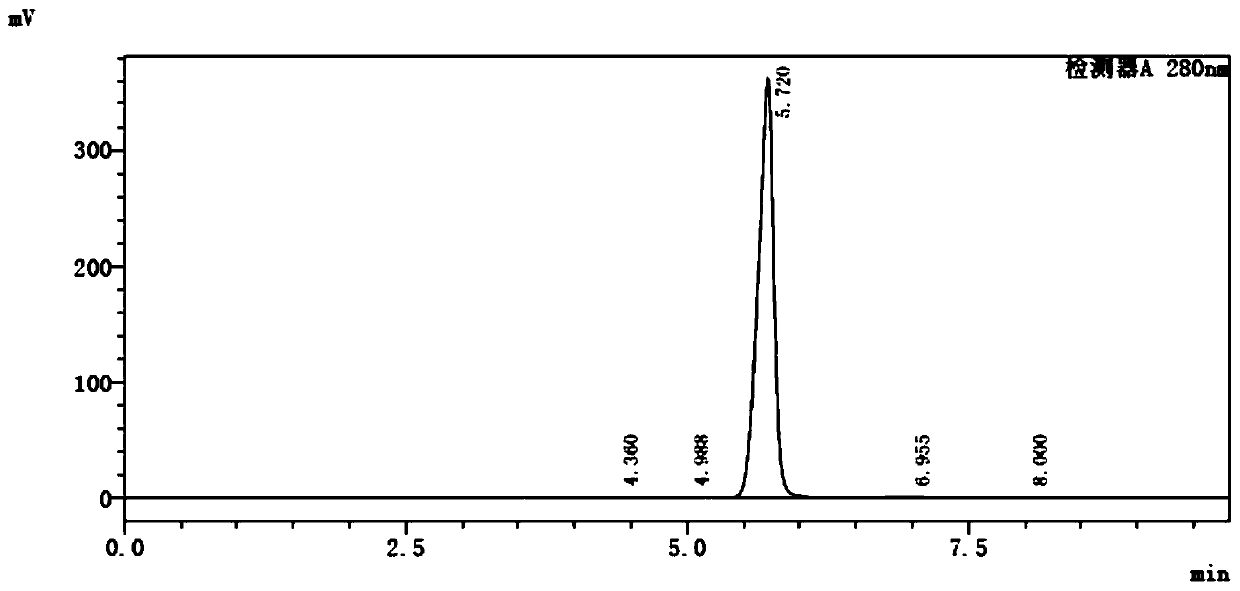
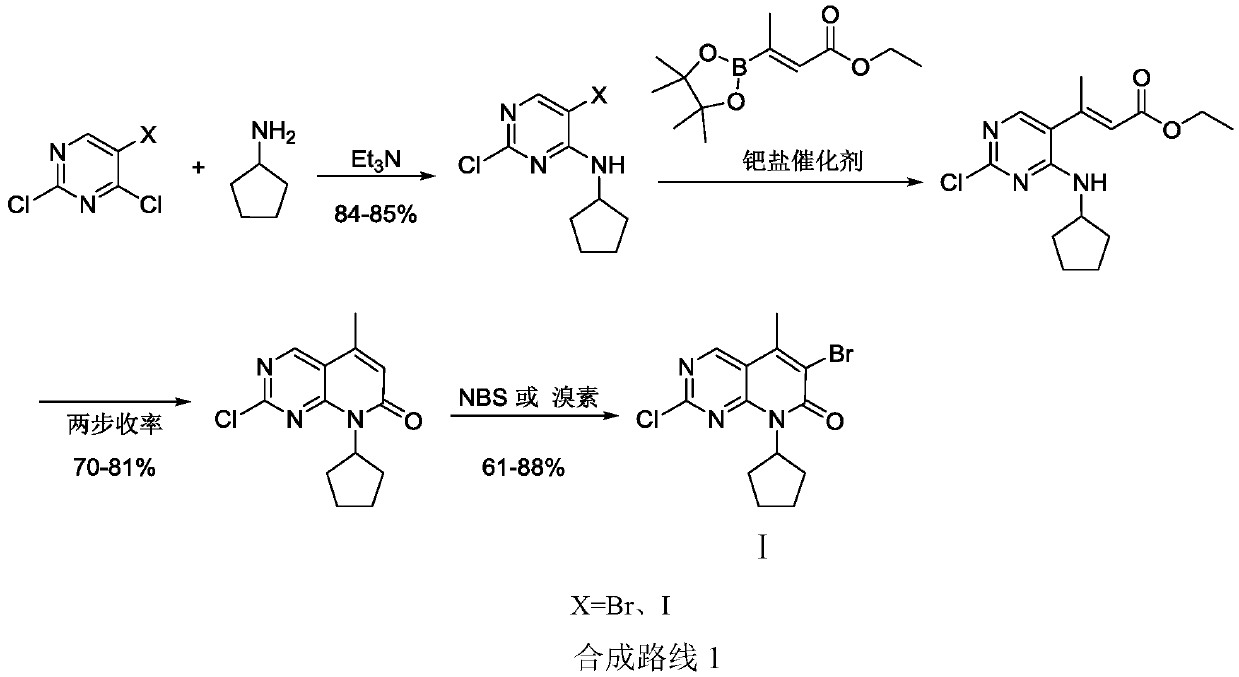
[0038]Example 1: Preparation of 2-chloro-5-methyl-6-bromo-8-cyclopentylpyridin[2,3-d]pyrimidin-8-hydrogen-7-one (I)
[0039] Step (1): Preparation of 2-hydroxy-5-methyl-8-cyclopentylpyridin[2,3-d]pyrimidin-8-hydrogen-7-one (Ⅴ)
[0040] In a 500 ml four-neck flask connected with a stirring, thermometer, and distillation device, add 120 g of N,N-dimethylformamide, 17.2 g (0.1 mole) of 3-methyl-2-pentaconate dimethyl , 8.6 g (0.1 mol) cyclopentylamine, 0.2 g DBU, 80 to 85 ° C stirring reaction for 4 hours, 110 to 115 ° C stirring reaction for 4 hours, while distilling off the generated methanol. Cool down to 50-60°C, add 14.3 g (0.12 moles) of N,N-dimethylformamide dimethyl acetal (DMF-DMA), react at 110 to 115°C for 5 hours, cool down to 50-60°C, add 10.0 g (0.17 mole) urea, reacted at 80 to 85°C for 5 hours, recovered part of the solvent (80-85 grams of solvent) by distillation under reduced pressure, lowered to room temperature, added 300 grams of water, filtered, and the fil...
Embodiment 2
[0052] Example 2: Preparation of 2-chloro-5-methyl-6-bromo-8-cyclopentylpyridin[2,3-d]pyrimidin-8-hydrogen-7-one (I)
[0053] Step (1): Preparation of 2-hydroxy-5-methyl-8-cyclopentylpyridin[2,3-d]pyrimidin-8-hydrogen-7-one (Ⅴ)
[0054] In a 500 ml four-necked flask connected with a stirring, thermometer, and distillation device, add 150 g of toluene, 20.0 g (0.1 mole) of 3-methyl-2-pentaconate diethyl ester, 8.6 g (0.1 mole) of cyclic Amylamine, 0.2 g of DBU, reacted with stirring at 80 to 85°C for 4 hours, and at 100 to 105°C for 4 hours, while distilling off the generated ethanol. Cool down to 50-60°C, add 14.3 g (0.12 moles) of N,N-dimethylformamide dimethyl acetal (DMF-DMA), react at 100 to 105°C for 7 hours, cool down to 50-60°C, add 10.0 g (0.17 moles) urea, reacted at 85 to 90°C for 7 hours, recovered the solvent by distillation under reduced pressure, cooled to room temperature, added 300 grams of water, filtered, washed the filter cake with 20 grams of isopropanol, ...
Embodiment 3
[0059] Example 3: Preparation of 2-chloro-5-methyl-6-bromo-8-cyclopentylpyridin[2,3-d]pyrimidin-8-hydrogen-7-one (I)
[0060] Step (1): Preparation of 2-hydroxy-5-methyl-8-cyclopentylpyridin[2,3-d]pyrimidin-8-hydrogen-7-one (Ⅴ)
[0061] In a 500 ml four-neck flask connected with a stirring, thermometer, and distillation device, add 120 g of N,N-dimethylformamide, 25.5 g (0.1 mole) of 3-methyl-2-pentaconate di-tert-butyl Esters, 8.6 g (0.1 mol) cyclopentylamine, 0.3 g DBU, stirred at 90 to 95° C. for 4 hours, and at 120 to 125° C. for 4 hours, while distilling off the generated tert-butanol alcohol. Cool down to 50-60°C, add 14.3 g (0.12 moles) of N,N-dimethylformamide dimethyl acetal (DMF-DMA), react at 110 to 115°C for 5 hours, cool down to 50-60°C, add 10.0 g (0.17 moles) urea, reacted at 90 to 95°C for 5 hours, recovered part of the solvent (80-85 grams of solvent) by distillation under reduced pressure, lowered to room temperature, added 300 grams of water, filtered, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com