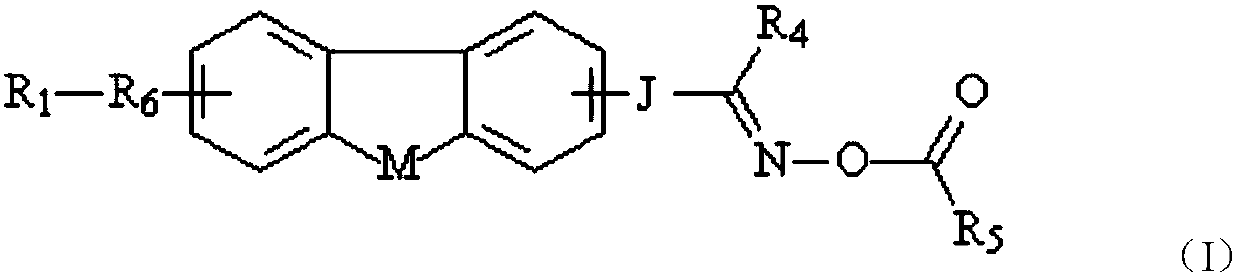
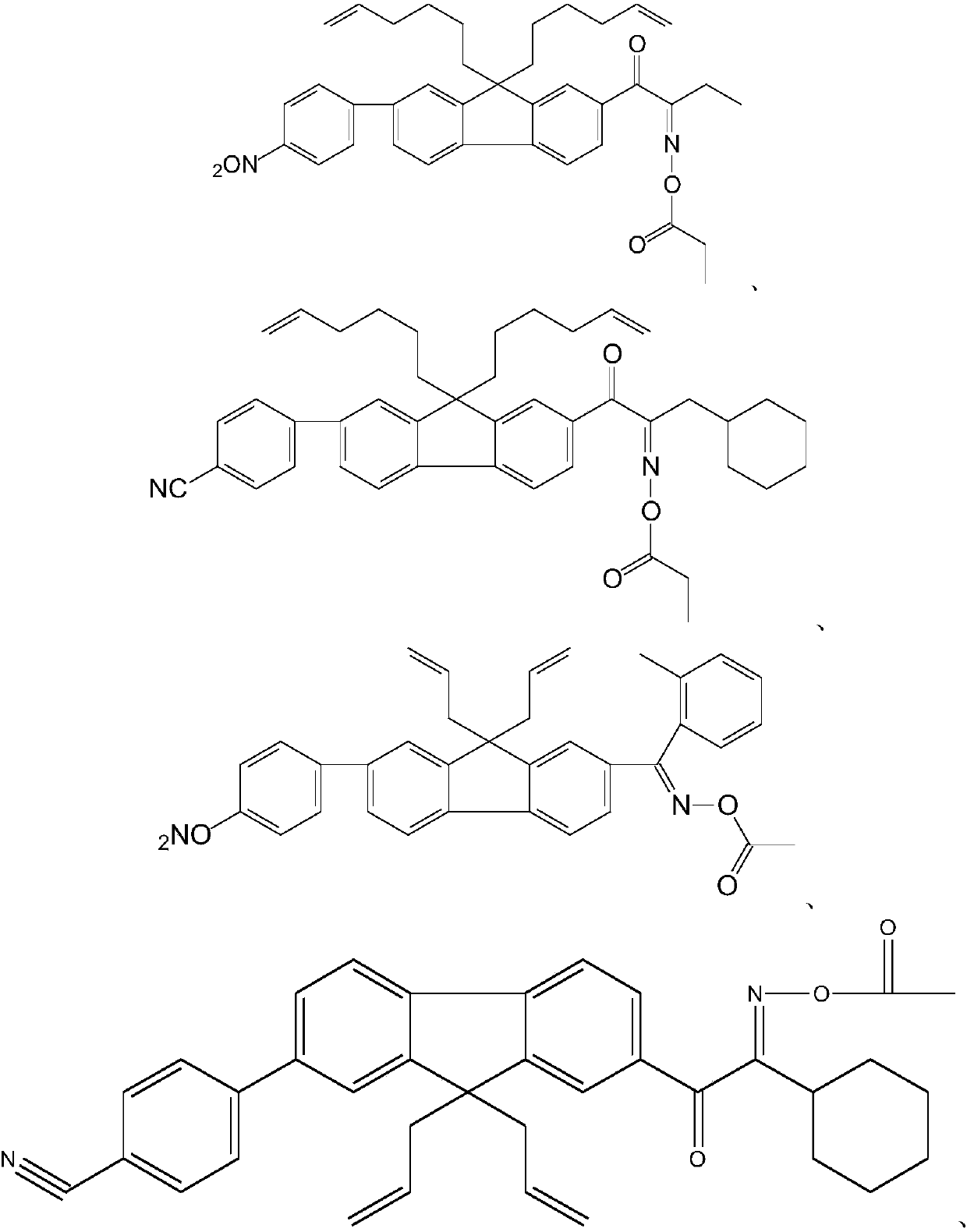
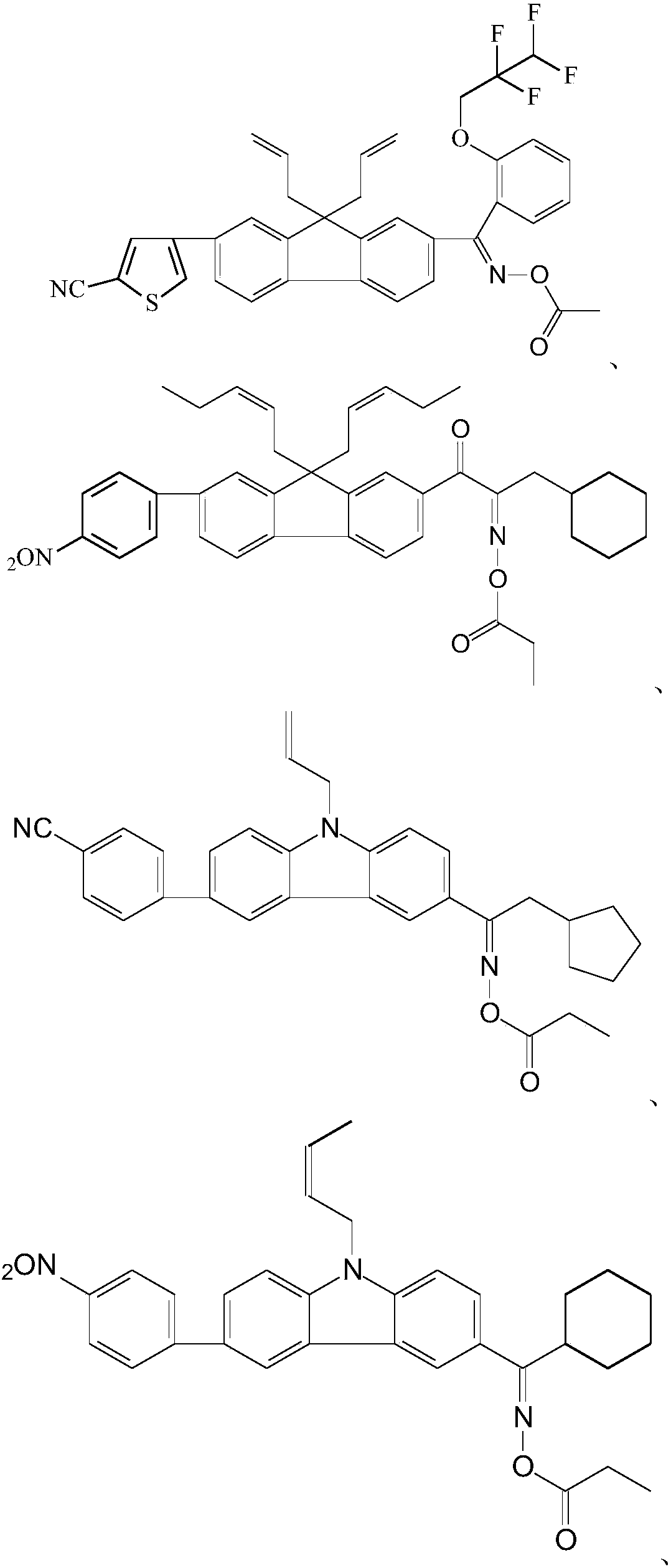
Oxime ester photoinitiator, preparation method, light-sensitive resin composition and application
A technology of photoinitiators and oxime esters, applied in the field of photocuring, can solve the problem of easy migration of photoinitiators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] In order to better achieve the above-mentioned purpose, another aspect of the present application also provides a preparation method of an oxime ester photoinitiator, the preparation method comprising:
[0064] S1, in the presence of the first catalyst, the raw material a and raw material b are subjected to Suzuki-Miyaura Cross Coupling (Suzuki-Miyaura Cross Coupling) to obtain intermediate a, the synthesis route is:
[0065]
[0066] R 1 Represents electron withdrawing group, R 4 Represents a monovalent organic group or hydrogen atom, R 6 One selected from an arylene group, a heteroarylene group, and a chain aliphatic hydrocarbon group containing at least one carbon-carbon double bond and / or at least one carbon-carbon triple bond, or the main chain end of the aforementioned aliphatic hydrocarbon group The group carbon atoms are respectively connected to the arylene group and / or heteroarylene group to form a group, or a group formed by connecting an arylene group and a hetero...
Embodiment 1
[0123] (1) Synthesis of Intermediate 1a
[0124] Into a 500mL four-necked flask was added 70.0g raw material 1a, 150mL tetrahydrofuran, 33.4g raw material 1b, 55.3g potassium carbonate, 3.2g (Pd(dppf)Cl 2 ·CH 2 Cl 2 ) And 15 mL of deionized water, heated to reflux at 70°C, and the liquid phase is tracked until the amount of raw material 1a no longer changes. Then the reaction system was cooled to room temperature, filtered to remove insoluble components, and then the filtrate was concentrated and dried, washed with water and recrystallized with ethyl acetate. Finally, the recrystallized product was dried in an oven at 80°C for 2 hours to obtain 59.6g of intermediate 1a. The rate is 76% by weight, and the purity is 98% by weight. The synthetic route is:
[0125]
[0126] Intermediate 1a was confirmed by proton nuclear magnetic resonance spectroscopy and mass spectrometry:
[0127] 1 H-NMR(CDCl 3 , 500MHz): 7.3006-7.3647 (3H, m), 7.4987-7.6235 (4H, m), 7.73976-7.7725 (4H, m), 7.9892-...
Embodiment 2
[0145] (1) Synthesis of intermediate 2a.
[0146] Add 73.9g of raw material 2a, 150mL of tetrahydrofuran, 29.4g of raw material 2b, 55.3g of potassium carbonate, 3.2g (Pd(dppf)Cl 2 ·CH 2 Cl 2 ) And 15mL of deionized water, heated to reflux at 70°C, the liquid phase is tracked until the amount of raw material 2a no longer changes, then the reaction solution is cooled to room temperature, filtered to remove insoluble components, then the filtrate is concentrated and dried, washed with water and recrystallized with acetone After drying in an oven at 80°C for 2 hours, 57.9 g of intermediate 2a was obtained, with a yield of 74 wt% and a purity of 98 wt%.
[0147] The synthetic route is:
[0148]
[0149] Intermediate 2a was confirmed by proton nuclear magnetic resonance and mass spectrometry:
[0150] 1 H-NMR(CDCl 3 , 500MHz): 1.4016 ~ 1.4448 (10H, m), 1.9688 ~ 1.9775 (1H, m), 2.4989 ~ 2.5206 (2H, dm), 3.8774 (2H, s), 7.5704 ~ 8.1935 (10H, m).
[0151] MS(m / z): 392(M+1) + .
[0152] (2) Synt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com