Synthesis method of laspeyresia pomonella sex pheromone
A synthesis method and technology of codling moth are applied in the field of synthesizing sex pheromone of codling moth, and can solve the problems of long time, high toxicity, and long route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Intermediate A:
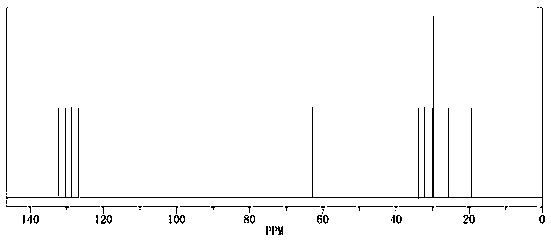
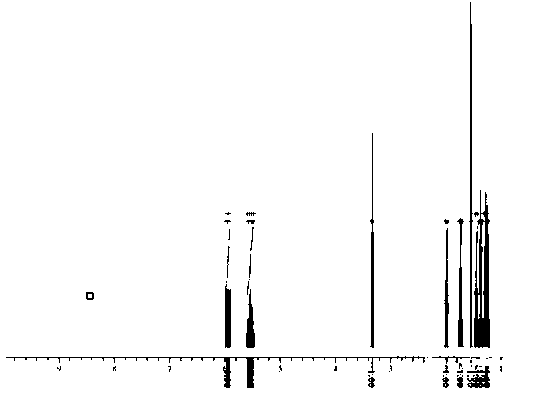
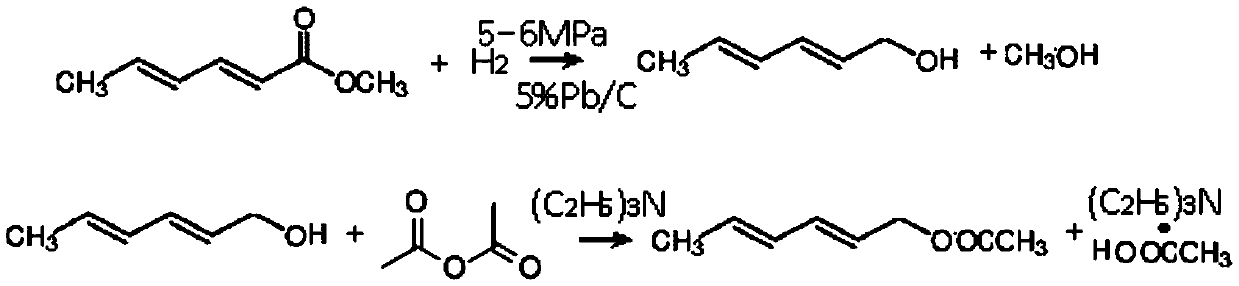
[0032] In a 500L dry and clean stainless steel autoclave, put 100Kg of methyl sorbate, 300Kg of methanol, and 300g of cat: 5% palladium carbon into a 500L dry and clean stainless steel autoclave, stir nitrogen replacement 3 times until there is no oxygen, heat up to 50°C, and pass in hydrogen To 6MPa, heat preservation and pressure reaction for 12h, until the reaction pressure no longer drops, cool down to 20°C, after pressure relief treatment, filter cat and activate it for reuse, concentrate the filtrate to recover methanol, and the remaining yellow oily liquid is 98.05% by GC detection . The liquid quantity is: 90.2kg, and the yield is 94.95%.
[0033] Intermediate B:
[0034] In a 500L dry and clean enamel reaction kettle, put 90.2kg of measured sorbitol, 200kg of ethyl acetate, 100kg of triethylamine, and 98kg of acetic anhydride, stir and raise the temperature to 55°C, stop the temperature rise, control 55°C, and stir the reaction After 12 hou...
Embodiment 2
[0040] Intermediate A:
[0041] In a 500L dry and clean stainless steel autoclave, put 100Kg of methyl sorbate, 200Kg of methanol, and 200g of cat: 5% palladium carbon into a 500L dry and clean stainless steel autoclave, stir nitrogen replacement 3 times until there is no oxygen, raise the temperature to 60°C, and pass in hydrogen To 5MPa, heat preservation and pressure reaction for 12h, until the reaction pressure no longer drops, cool down to 20°C, after the pressure relief treatment, filter the cat and activate it for reuse, concentrate the filtrate to recover methanol, and the remaining yellow oily liquid is 97.15% by GC detection . The amount of liquid is: 88.2kg, and the yield is 92.84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com