Cationic chain transfer agent and application thereof
A technology of chain transfer agent and chain transfer reagent, which is applied in the field of organic catalysis and polymer materials, can solve the problems of low raw material cost, low degree of polymerization, low molecular weight, and inability to prepare block copolymers, and achieve controllable process and molecular weight distribution. Narrow, Molecular Weight Controlled Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
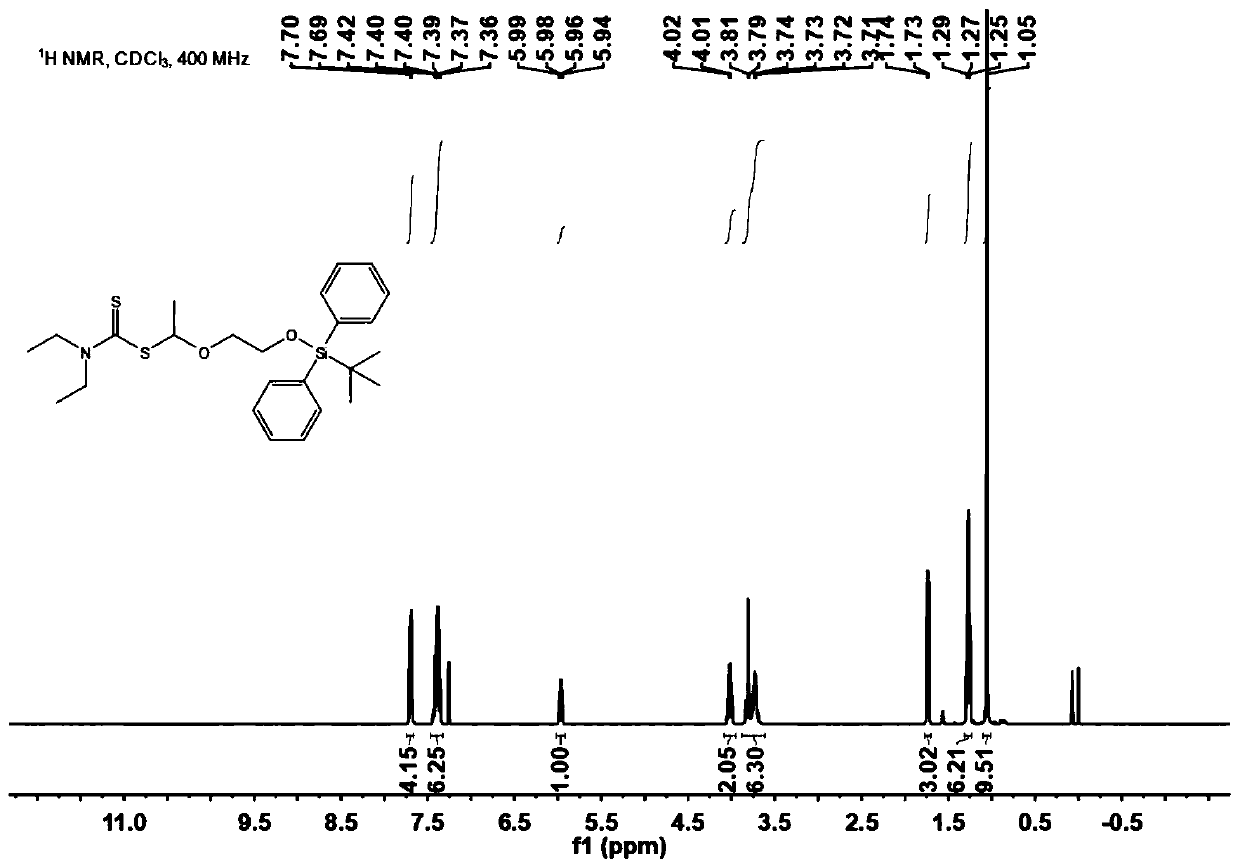
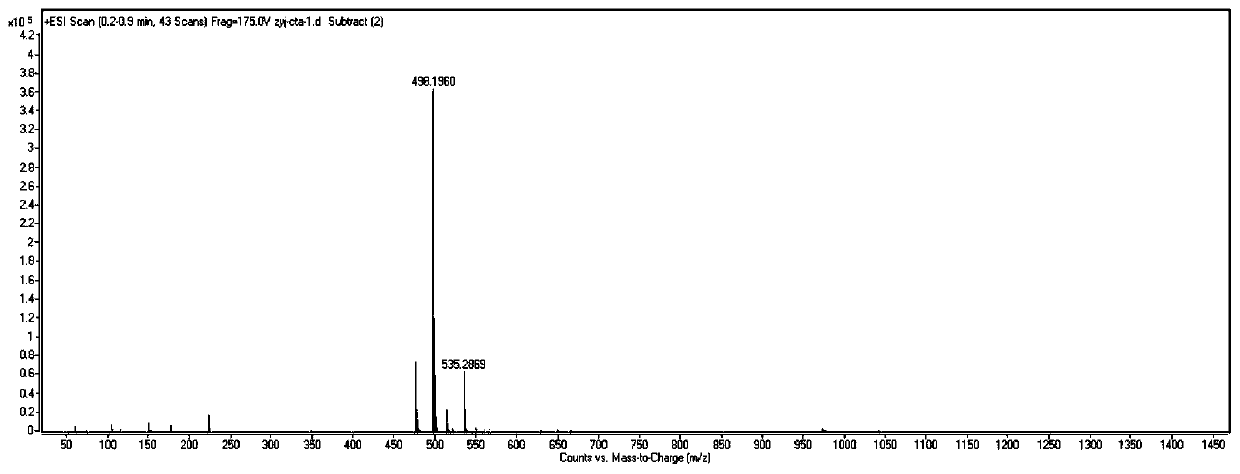
[0064] To DMF (30 mL) containing 2.2 equivalents of imidazole (1.513 g, 0.022 mmol) was added 1.1 equivalents of tert-butyldiphenylchlorosilane (2.92 mL, 0.011 mmol) and ethylene glycol monovinyl ether (0.92 mL, 0.01 mmol). Under the condition of 25°C, the reaction was stirred for 4 hours. The completion of the reaction was detected by spotting the plate, and purification was carried out by column chromatography. At -78°C, 1.0M HCl ether solution (88mL, 88mmol) was added dropwise to the purified substance (19.0mL, 80mmol) in ether solution (250mL), reacted for one hour, and the solvent was evaporated off. To a solution of the amino-substituted sodium dithiocarbamate (9.80 g, 40 mmol) in ether was added a solution of the halogenated product (30 mmol) in a slight excess of hydrogen chloride dropwise over 30 minutes at 0°C. After stirring at 0°C for 1 hour and at room temperature for 1.5 hours, the reaction was diluted with ether to quench the reaction. Then, the solution was ...
Embodiment 2
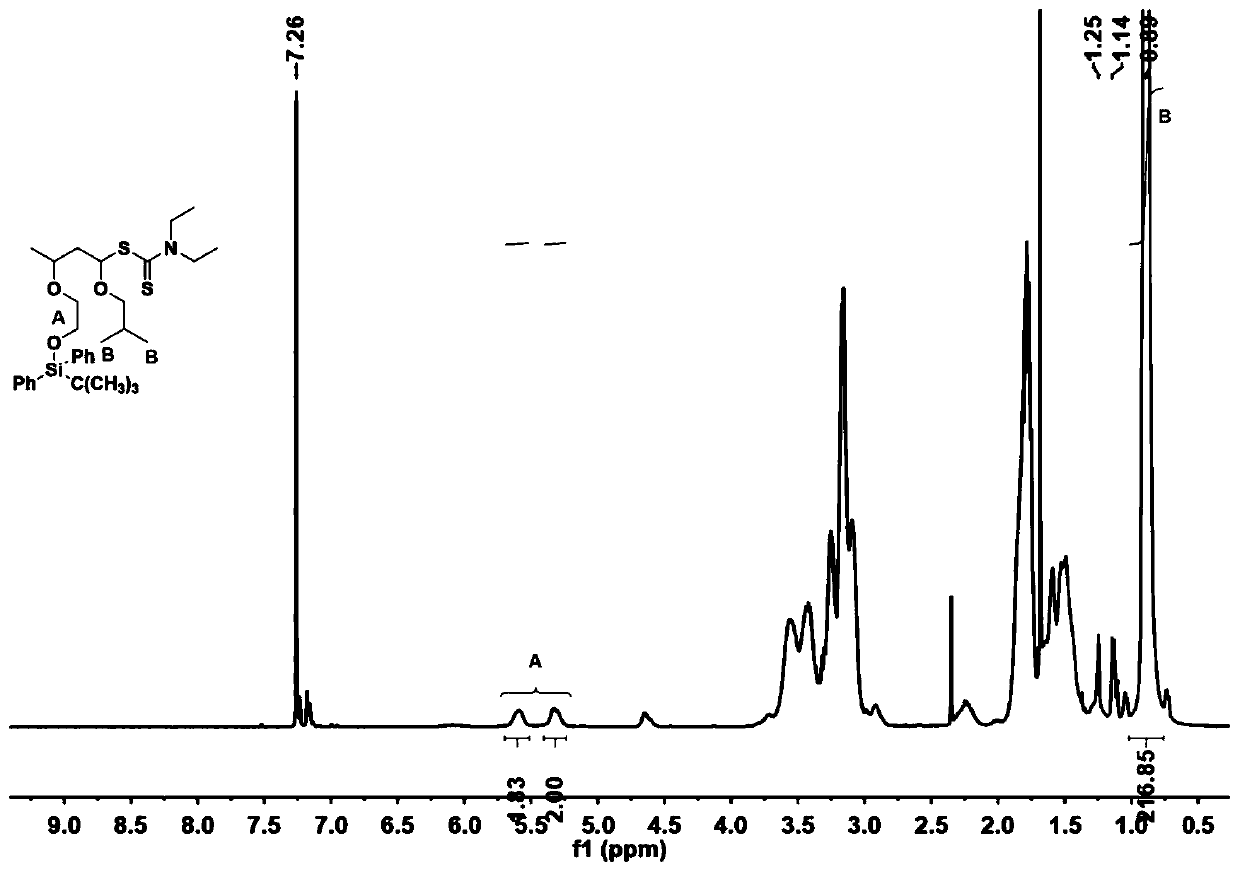
[0067] Under a nitrogen atmosphere, add a cationic chain transfer agent (0.03mM, the synthesis steps are the same as in Example 1) with a structure as shown in No. 1, and an isobutyl vinyl ether with a structure as shown in No. 35 into the baked polymerization tube (1.53mM), toluene (0.05mL) in n-hexane, dichloromethane (8:1 volume ratio) mixed solution (2.7mL), the molar ratio of monomer and cationic chain transfer agent is 50:1, and then through the dry syringe A solution of trifluoromethanesulfonic acid in ether (0.50 mM, 0.30 mL) was added. Perform three freeze-pump-thaw cycles to remove the oxygen in the reaction system, seal the nitrogen atmosphere, react at -40°C for 90 minutes, and the conversion rate is greater than 99%. The number average molecular weight Mn of the obtained polymer is 4.9kg / mol, and the molecular weight distribution PDI is 1.26. Then, tetra-n-butylammonium fluoride was added and reacted at room temperature for 5 hours to release the terminal hydroxy...
Embodiment 3
[0069] Under a nitrogen atmosphere, add a cationic chain transfer agent (0.03mM, the synthesis steps are the same as in Example 1) with a structure as shown in No. 1, and an isobutyl vinyl ether with a structure as shown in No. 35 into the baked polymerization tube (1.53mM), toluene (0.05mL) in n-hexane, dichloromethane (8:1 volume ratio) mixed solution (2.7mL), the molar ratio of monomer and cationic chain transfer agent is 50:1, and then through the dry syringe A solution of trifluoromethanesulfonic acid in ether (0.50 mM, 0.30 mL) was added. Perform three freezing-pumping-thawing cycles to remove the oxygen in the reaction system, seal the nitrogen atmosphere, react at -40°C for 90 minutes, and the conversion rate is greater than 99%. The number average molecular weight Mn of the obtained polymer is 5.0kg / mol, and the molecular weight distribution PDI is 1.19. Then, tetra-n-butylammonium fluoride was added and reacted at room temperature for 5 hours to release the terminal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com