Ruthenium catalyst for synthesis ammonia and preparation method thereof
A ruthenium catalyst and a technology for synthesizing ammonia, applied in catalyst activation/preparation, ammonia preparation/separation, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
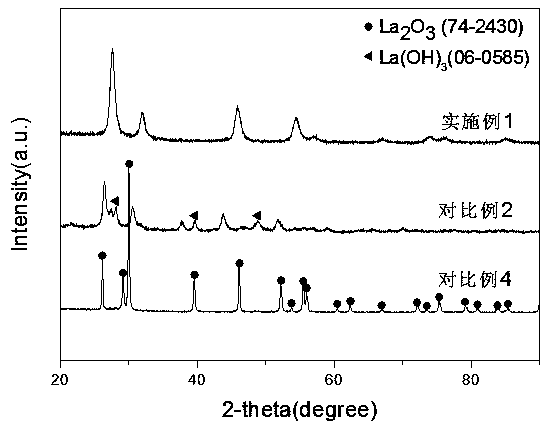
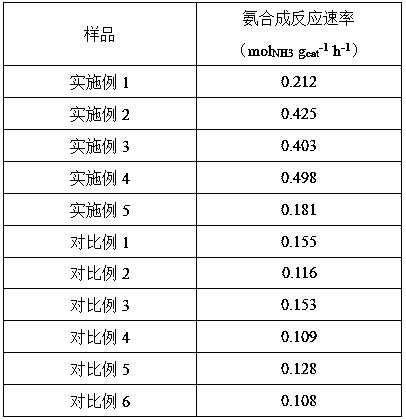
[0022] 1) Dissolve 6.46 g of lanthanum nitrate and 6.48 g of cerium nitrate in 10 mL of water to form an aqueous solution; then add 70 mL of 25% ammonia water to the aqueous solution and stir for 1 hour to form a precipitate. After the obtained precipitate was dried, the temperature was raised to 700 °C at 1 °C / min under an oxygen atmosphere, and calcined for 5 hours to obtain CeO with a La:Ce molar ratio of 1:1. 2 -La 2 o 3 composite oxides.
[0023] 2) The ruthenium nitrosyl nitrate was dried at 80 °C for 3 h and then dissolved into a methanol solution with a volume concentration of 75% to obtain a ruthenium precursor alcohol solution;
[0024] 3) The ruthenium precursor alcohol solution obtained in step 2) was impregnated into the lanthanum-cerium composite support prepared in step 1) at 30 °C for 1 h.
[0025] 4) Dry the sample prepared in step 3) at 100 °C for 0.5 h, and then reduce it with pure hydrogen at 400 °C for 2 h to obtain the ruthenium-based ammonia synthesis...
Embodiment 2
[0027] 1) Dissolve 4.16 g of lanthanum nitrate and 9.73 g of cerium nitrate in 10 mL of water to form an aqueous solution; then add 50 mL of potassium hydroxide solution (5mol / L) to the aqueous solution and stir for 1 hour to form a precipitate. After the obtained precipitate was dried, the temperature was raised to 600 °C at 10 °C / min under an oxygen atmosphere, and calcined for 4 hours to obtain CeO with a La:Ce molar ratio of 3:7. 2 -La 2 o 3 composite oxides.
[0028] 2) After drying ruthenium carbonyl at 60 °C for 6 h, it was dissolved in a methanol solution with a volume concentration of 60% to obtain a ruthenium precursor alcohol solution;
[0029] 3) The ruthenium precursor alcohol solution obtained in step 2) was impregnated into the lanthanum-cerium composite support prepared in step 1) at 30 °C for 2 h.
[0030] 4) Dry the sample prepared in step 3) at 150 °C for 1 h, and then 2 -Ar mixed gas was reduced at 550 °C for 4 h to obtain the ruthenium-based ammonia sy...
Embodiment 3
[0032] 1) Dissolve 5.54 g of lanthanum nitrate and 8.34 g of cerium nitrate in 10 mL of water to form an aqueous solution, then add 85 mL of 3 mol / L urea solution to the aqueous solution and stir for 0.5 hours to form a precipitate. After the obtained precipitate was dried, the temperature was raised to 500 °C at 5 °C / min under an oxygen atmosphere, and calcined for 4 hours to obtain CeO with a La:Ce molar ratio of 2:3. 2 -La 2 o 3 composite oxides.
[0033] 2) After drying ruthenium carbonyl at 80 °C for 3 h, it was dissolved in absolute ethanol solution to obtain ruthenium precursor alcohol solution;
[0034] 3) The ruthenium precursor alcohol solution obtained in step 2) was impregnated into the lanthanum-cerium composite support prepared in step 1) at 25 °C for 0.5 h.
[0035] 4) The sample prepared in step 3) was dried at 90 °C for 1 h, and then washed with 10% H 2-Ar mixed gas was reduced at 550 °C for 3 h to obtain the ruthenium-based ammonia synthesis catalyst, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com