Preparation of confinement structure ruthenium-nickel core-shell bimetallic nano-catalyst and application thereof in catalyzing dimethyl terephthalate selective hydrogenation
A bimetallic nanometer and metal nanoparticle technology, which is applied in the direction of metal/metal oxide/metal hydroxide catalyst, organic compound preparation, hydroxyl compound preparation, etc., can solve the problem of poor catalyst activity and life, harsh reaction conditions, and price Expensive and other issues, to achieve the effect of improving conversion rate, simple method, and improving reaction performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
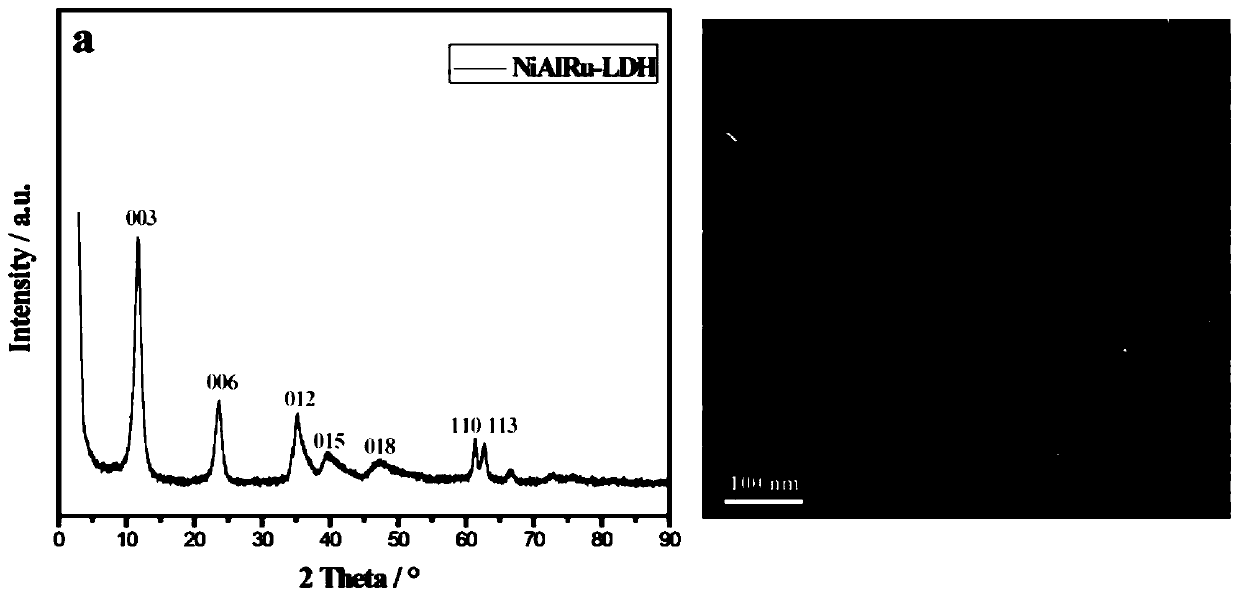
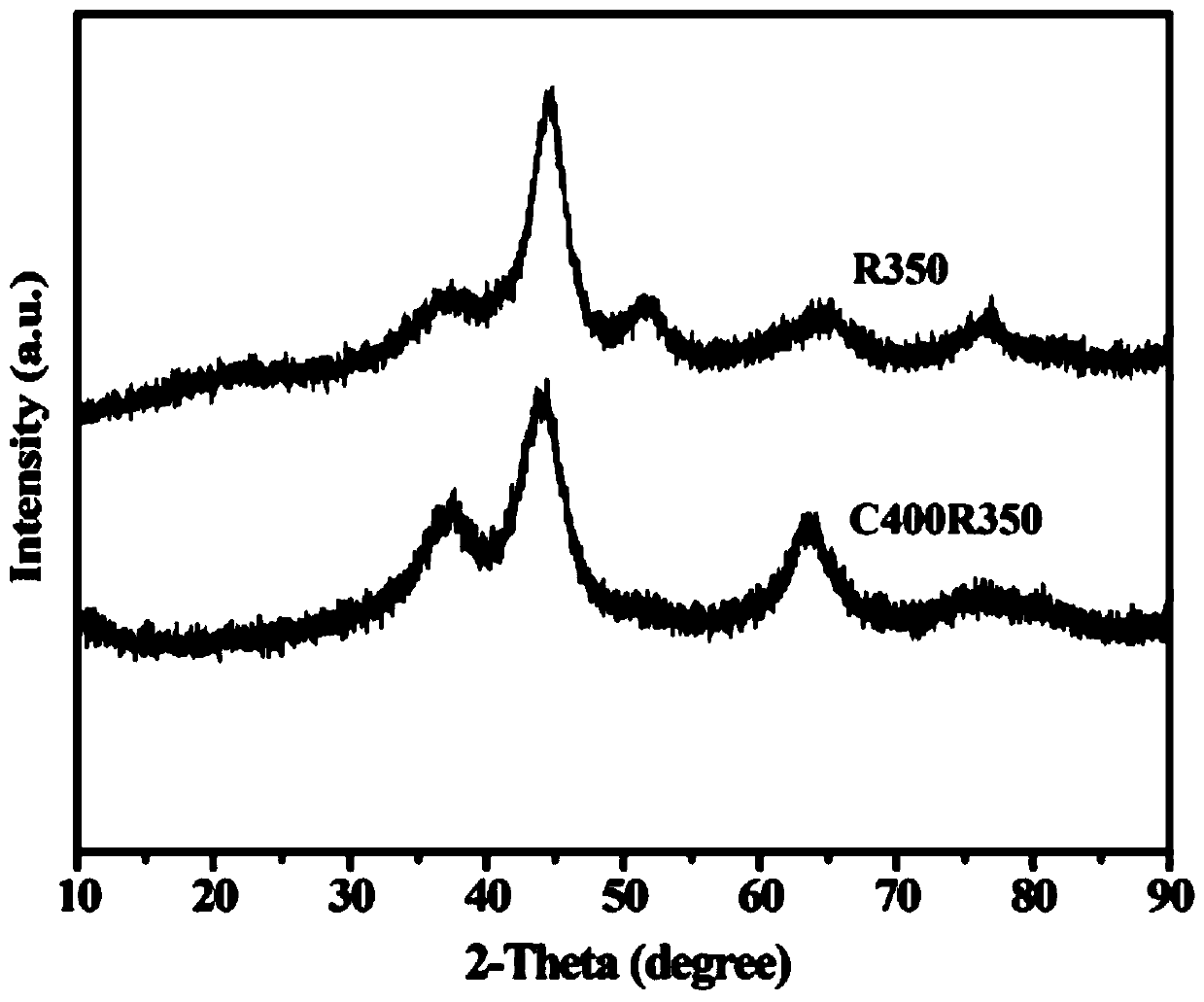
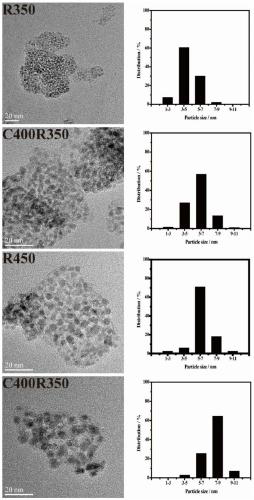
[0019] A. Preparation of NiAlRu-LDH: Weigh 12.79g of Ni(NO 3 ) 2 ·6H 2 O and 8.25g of Al(NO 3 ) 2 9H 2 O was dissolved in 77mL of deionized water and added to 23mL with a molar concentration of 0.00241mol·mL -1 RuCl 3 Salt solution, prepared as a Ni / Al molar ratio of 2, Ni / Ru molar ratio of 100 mixed salt solution; Weigh 5.49g of sodium hydroxide and 6.06g of sodium carbonate into 130mL of deionized water, ultrasonically dissolved to obtain a mixed alkali solution. Measure 100 mL of deionized water into the synthesis system. At room temperature, the mixed salt solution and the mixed alkali solution were added dropwise to a four-necked flask filled with 100 mL deionized water through a double-channel micro-injection pump, and the dropping rate of the salt solution was 20 mL h -1 , maintain a constant pH = 10, and crystallize at 120°C for 24h. Then wash with deionized water at about 60°C until neutral, and finally dry at 70°C for 24 hours to obtain a highly dispersed hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com