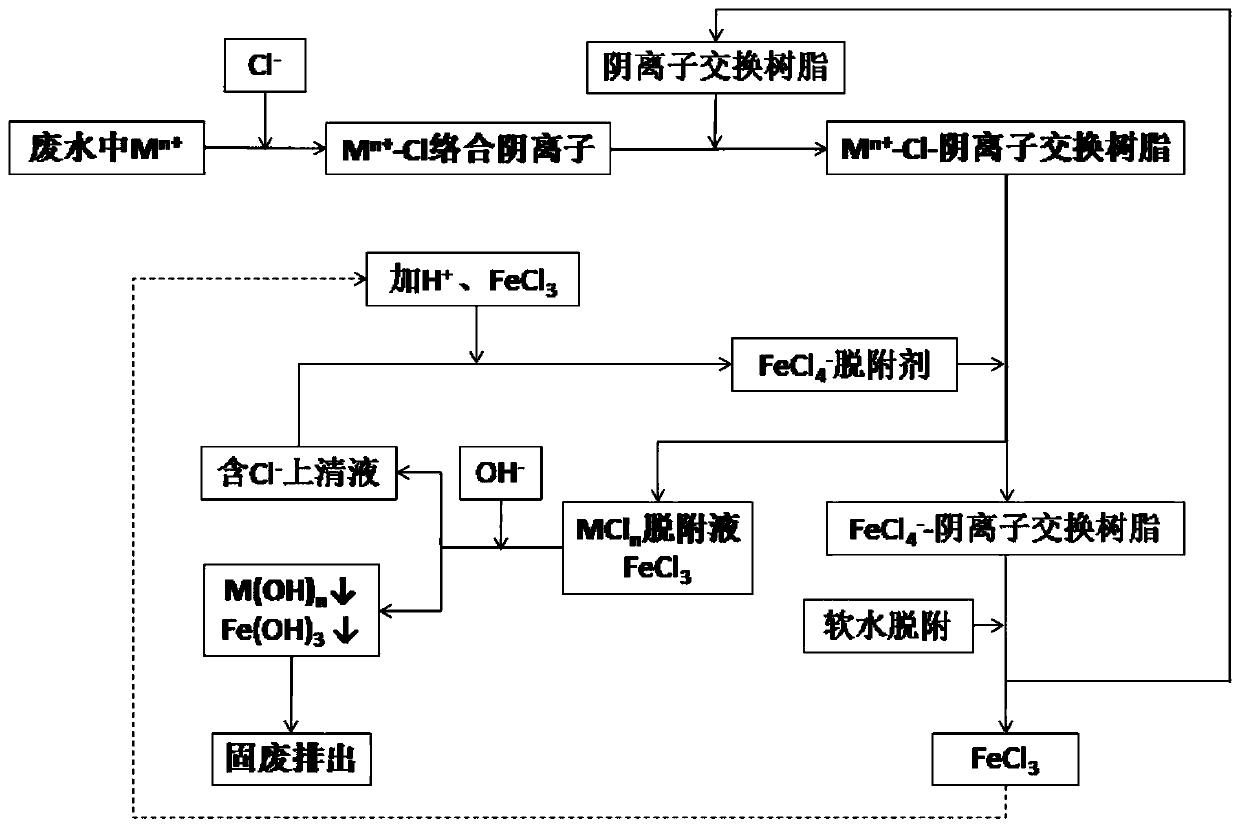
Method for removing heavy metal ions in wastewater through ion exchange resin and resin regeneration method
A technology of ion exchange resin and heavy metal ions, which is applied in ion exchange water/sewage treatment, ion exchange bed cleaning/rinsing, chemical instruments and methods, etc., can solve the problem of high cost of heavy metal ions, reduce the consumption of chemicals, and facilitate fixing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
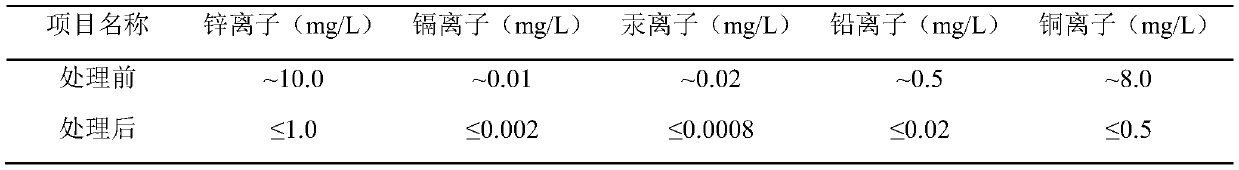
[0046] 20mL strong basic anion exchange resin (NDA-900, specific surface is 20.0~100.0m 2 / g, the exchange capacity is 0.5~2.5mmol / g, Jiangsu Nanda Environmental Protection Technology Co., Ltd.) into a jacketed glass adsorption column (Φ32×360mm). At room temperature (20.0° C.), chloride ions were added to the solution containing heavy metal ions such as zinc, cadmium, mercury, lead, and copper (see Table 1) after precipitation pretreatment (adjusting the mass percentage of chlorine in the mixed solution to be 10%) ) to make heavy metal ions all form complex anions with chloride ions, pass the waste water through the NDA-900 bed at a flow rate of 1.0BV / h, and the waste water treatment capacity is at least 3500.0BV / batch, and the specific inlet and outlet water quality in embodiment 1 The situation is shown in Table 1.
[0047] Table 1 Resin column inlet and outlet water quality
[0048]
[0049]
[0050] Note: The reference implementation standard in the above table is...
Embodiment 2
[0053] Put 20mL of strongly basic anion exchange resin (NDA-900) into a jacketed glass adsorption column (Φ32×360 mm). In addition to heavy metal ions, wastewater also contains Na + Concentration greater than 10mg / L, Ca 2+ The concentration is greater than 10mg / L. At room temperature (20.0°C), adjust the chloride ion concentration in the wastewater containing zinc, cadmium, mercury, lead, copper and other heavy metal ions and sodium and calcium ions after precipitation pretreatment, so that the mixed solution The mass percent content of chlorine is 8%, and the wastewater that forms complex anions passes through the NDA-900 bed at a flow rate of 3.0BV / h, and the wastewater treatment capacity is at least 3500.0BV / batch. The specific inlet and outlet water quality in embodiment 2 The situation is shown in Table 2.
[0054] Table 2 Resin column inlet and outlet water quality
[0055]
[0056] Note: The reference implementation standard in the above table is the Class IV wate...
Embodiment 3
[0059] Adsorption treatment adopts the same process as Example 1, and results similar to Example 1 can be obtained.
[0060] Using FeCl 3 , NaCl, hydrochloric acid reagents to prepare FeCl 4 - Desorbing agent, the content of iron element in the desorbing agent is FeCl 3 The calculation is that the volume fraction is about 5.0%; the NaCl mass fraction is about 5.5%; the pH value is about 3.0, using 3.0BV compound desorption agent + 2.0BV demineralized water in turn, passing through the above NDA at a flow rate of 2BV / h at 30°C The -900 bed is desorbed and washed for regeneration, and the desorption rate of complex metal ions reaches 99.0%; the resin regeneration rate after soft water desorption reaches more than 99%. The high-concentration desorption solution contains a large amount of ions of metal elements such as zinc, cadmium, mercury, lead, copper and iron, and adjusts the pH value of the solution to 8-12, so that the ions of the aforementioned heavy metal elements form...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com