Lipid binding protein-antigen capturing module compound and preparing method and application thereof
A technology for binding proteins and complexes, applied in the biological field, which can solve the problems of extraction of membrane proteins, low assembly efficiency, unsatisfactory uniformity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0201] Embodiment 1: the expression of membrane protein on living cell (prokaryotic cell):
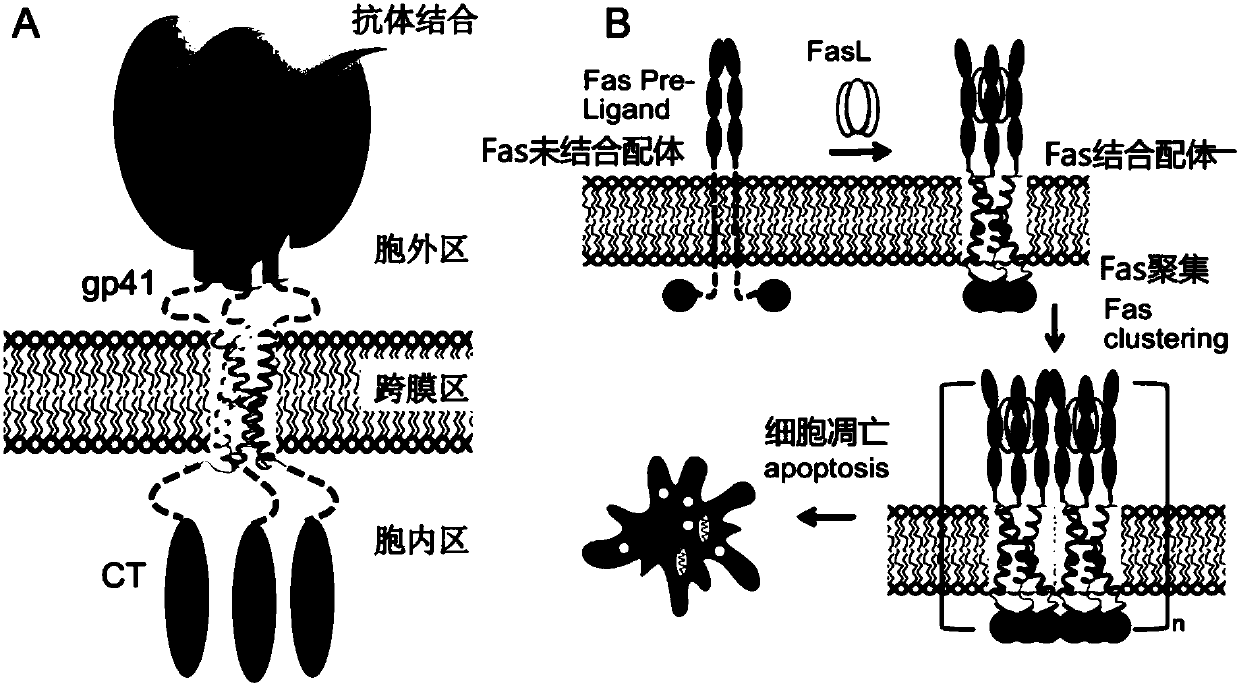
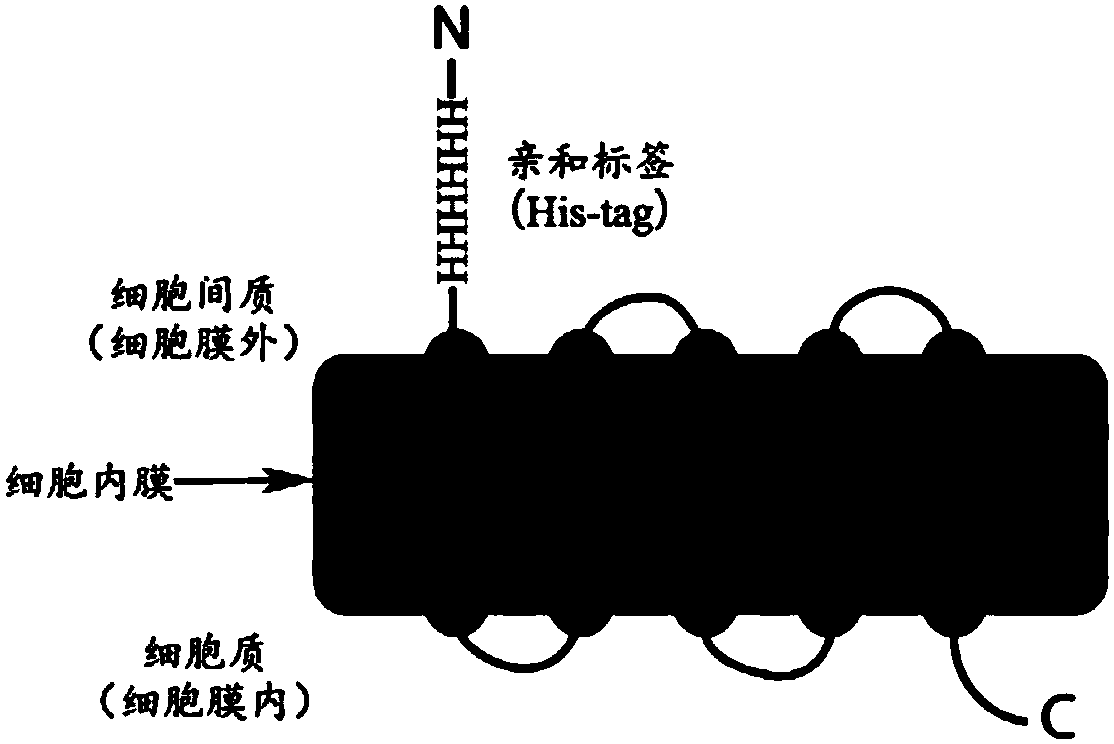
[0202] For the expression of membrane proteins on the surface of prokaryotic cells, Escherichia coli cells are used as an example to express TSPO (Translocator protein) membrane proteins on their cell membranes. According to literature reports, TSPO protein is expressed on the inner membrane of Escherichia coli, and its N-terminus is outside the membrane, while the C-terminus is inside the membrane (see image 3 ). Therefore, a His-tag (HHHHHH) is added to the N-terminus of the TSPO protein, and its final amino acid sequence is as follows:
[0203] HHHHHHNMDWALFLTFLAASGAPATTGALLKPDEWYDNLNKPWWNPPRWVFPLAWTSLYFLMSLAAMRVAQLEGSGQALAFYAAQLAFNTLWTPVFFGMKRMATALAVVMVMWLFVAATMWAFFQLDTWAGVLFVPYLIWATAATGLNFEAMRLN (SEQ ID NO. 6)
[0204] The His-TSPO gene was optimized to the preferred codon sequence of Escherichia coli, and after the sequence was artificially synthesized, it was cloned between t...
Embodiment 2
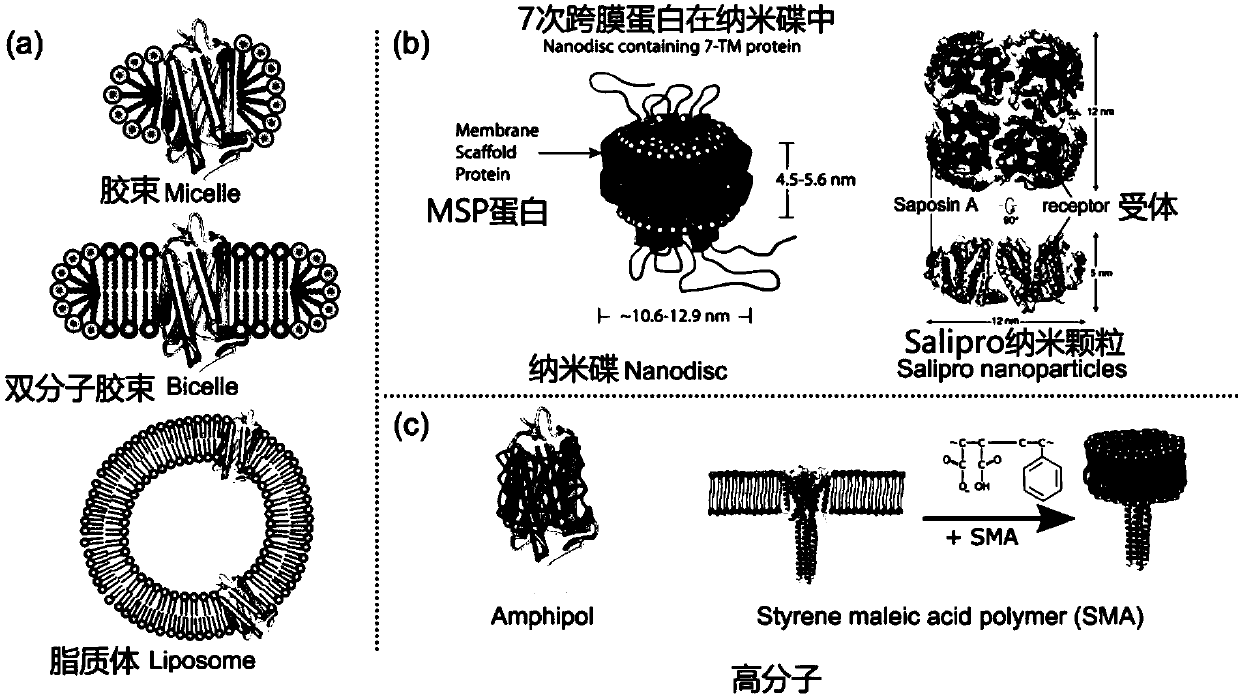
[0206] Example 2: Preparation of nanodisk "framework protein" MSP and Saposin
[0207] For the MSP protein, its wild-type and cysteine mutants need to be prepared separately. See SEQ ID NO.5 for the full-length amino acid sequence of wild-type MSP, and see SEQ ID NO.1 and SEQ ID NO.2 for the amino acid sequences of MSP cysteine mutants.
[0208] MGSSHHHHHHENLYFQGSTFSKLREQLGPVTQEFWDNLEKETEGLRQEM S KDLEEVK A KVQPYLDDFQKKWQEEMELYRQKVEPLRAELQEGARQKLHELQEKLSPLGEEMRDRARAHVDALRTHLAPYSDELRQRLAARLEALKENGGARLAEYHAKATEHLSTLSEKAKPALEDLRQGLLPVLESFKVSFLSALEEYTKKLNTQ (SEQ ID NO. 5)
[0209] SEQ ID NO.1 MSP cysteine mutant S86C amino acid sequence (wherein, the mutated C is underlined and located at position 50)
[0210] MGSSHHHHHHENLYFQGSTFSKLREQLGPVTQEFWDNLEKETEGLRQEM C KDLEEVKAKVQPYLDDFQKKWQEEMELYRQKVEPLRAELQEGARQKLHELQEKLSPLGEEMRDRARAHVDALRTHLAPYSDELRQRLAARLEALKENGGARLAEYHAKATEHLSTLSEKAKPALEDLRQGLLPVLESFKVSFLSALEEYTKKLNTQ
[0211] SEQ ID NO.2 MSP cysteine mutant A95C amino ac...
Embodiment 3
[0224] Embodiment 3: the preparation of affinity probe
[0225] Taking His-tag as an example for the affinity tag, the affinity probe can be a small molecule with high affinity to His-tag, such as tri-NTA, or a high-affinity protein macromolecule such as anti-His-tag scFv.
[0226] (1) Synthesis of Tri-NTA
[0227] For the synthetic route of Tri-NTA see Figure 5 .
[0228] First synthesize compound 1: tert-Butyl bromoacetate (tert-Butyl bromoacetate) (3ml, 20mmol) and EDIAP (4.3ml, 25mmol) were added in the DMF solution (40ml) of compound 0 (1.65g, 5mmol), under nitrogen Heated to 55°C under deoxygenation conditions and stirred continuously overnight. The reaction solution was heated to 60°C to remove volatile matter, and then 20ml of ethyl acetate was added and filtered. The precipitate was washed three more times with ethyl acetate. The filtrate was collected and concentrated. Purify with a silica gel column, and the mobile phase is cyclohexane / ethylacetate (3:1), to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com