Method for detecting related substances in Ilaprazole drug
A related substance, ilaprazole technology, applied in the field of detecting related substances in ilaprazole drugs, can solve the problems of drug stability, side reactions, high toxicity and the like that endanger human health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Get an appropriate amount of ilaprazole tablet fine powder (approximately equivalent to ilaprazole 15mg), put it in a 50ml measuring bottle, add solvent (600ml of 0.01mol / L dipotassium hydrogen phosphate solution and 400ml of acetonitrile and mix with 1mol / L hydroxide Sodium solution to adjust the pH value to 11.0 mixed solution, hereinafter referred to as solvent A) Appropriate amount, alternately dissolve by ultrasonic and shaking, each ultrasonic time is no more than 5 seconds, repeat 8-10 times, add solvent to dilute to the mark , shake well, filter, and take the continued filtrate as the test solution;
[0087] Precisely measure 1ml of the test solution, put it in a 100ml measuring bottle, dilute it to the mark with solvent A, shake it up, and use it as a reference solution; take 2-[[(4-methoxy-3-methyl)-2-pyridine Base]methyl]-sulfonyl-5-(1H-pyrrol-1-yl)-1H-benzimidazole (ilaprazole sulfone) about 5 mg, put it in a 100ml measuring bottle, add solvent A to dissolve...
Embodiment 2
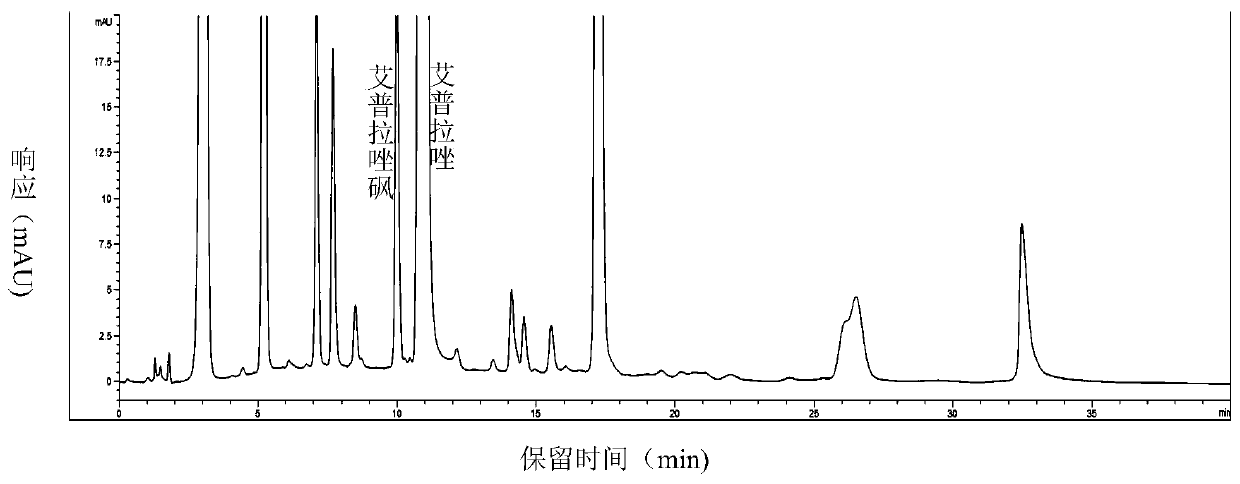
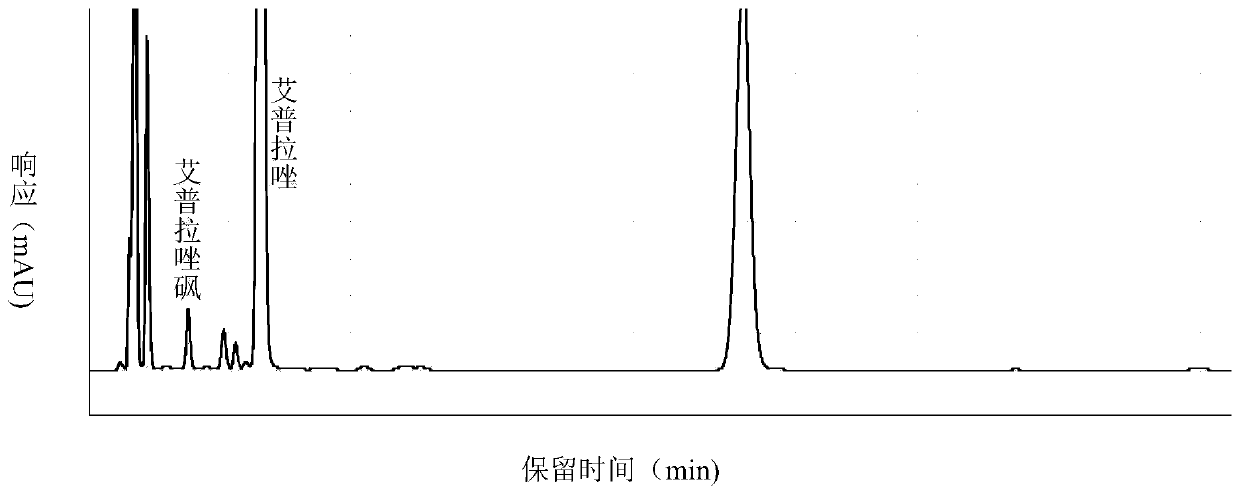
[0100] Get 3 batches of ilaprazole tablets, each batch gets 2 groups, according to the method of embodiment 1 and comparative example 1 respectively, the detection of related substances is carried out to the test solution, and the results are shown in table 2.
[0101] Table 2 Test results of related substances
[0102]
[0103]Note: T means retention time (min), N.D means not detected.
[0104] The detection results show that under the same batch, the detection method of Example 1 detects more impurities and the number of impurities than Comparative Example 1, and has higher sensitivity, and is more suitable for the detection of related substances of the ilaprazole pharmaceutical composition.
Embodiment 3
[0105] Embodiment 3 chromatographic column screening
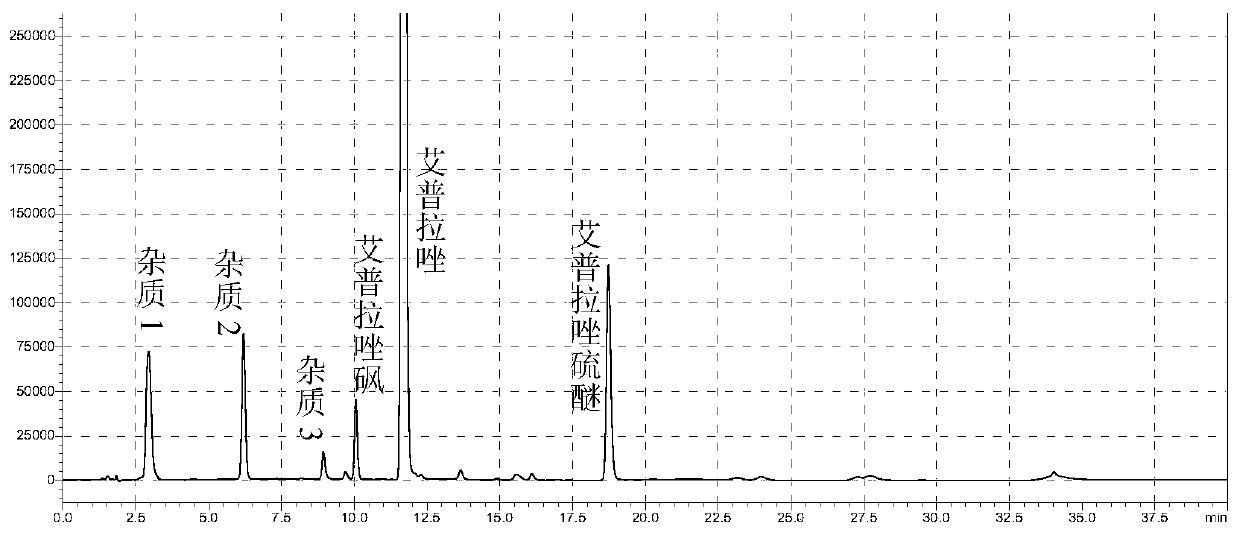
[0106] Get the test solution prepared in Example 1 and put it in a 90°C water bath to destroy it, and add 1ml of the ilaprazole sulfone reference substance solution prepared in Example 1, mix well, and use the mixed solution as the sample solution, according to Example 1 The method detects the related substance of sample solution, when detecting, uses different chromatographic columns (as shown in table 3), and detection result is shown in table 3.
[0107] Table 3 Test results of different chromatographic columns
[0108]
[0109] Note: R means resolution, T means retention time (min).
[0110] The results showed that when Gemini-NX C18 5μm (150×4.6mm), ACE Excel5 Super C18 1.7μm (150*4.6mm) and ECOSIL 120-5-C18 SH 5.0μm (150*4.6mm) were used as chromatographic columns, the impurities Most of the separation between the main peak and between impurities meets the requirements, and the number of detected impurities is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com