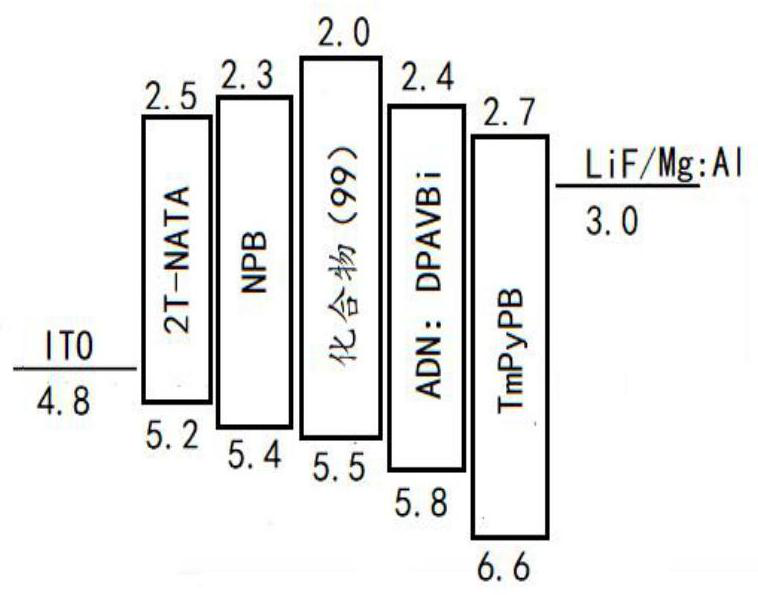
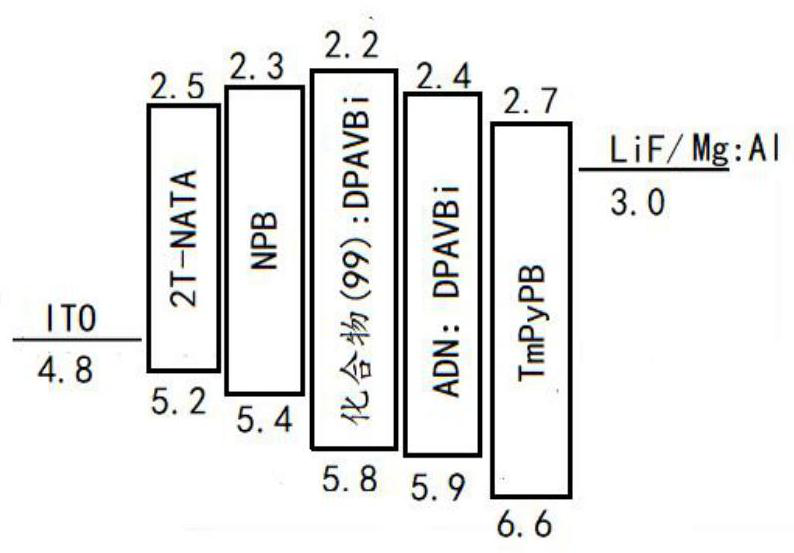
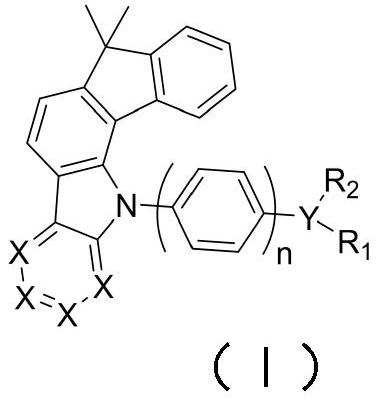
A kind of fluorenocarbazole derivative, its preparation method and application
A technology for fluorenocarbazole and derivatives, which is applied in the fields of fluorenocarbazole derivatives, their preparation and application, can solve the problems of restriction and difficult synthesis, and achieves high product yield and purity, and uniform product yield and purity. , synthesizing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the synthesis of compound (1)
[0055] 1a. Synthesis of fluorenocarbazole:
[0056]
[0057] S1. In a 250mL three-necked flask, put 4-bromo-9,9-dimethyl-9H-fluorene (8.20g, 30 mmol), 2-chloroaniline (4.59g, 36mmol), triphenylphosphine (0.04 g, 0.15mmol), sodium tert-butoxide (8.65g, 90mmol), add 80mL ethanol and 40mL water, under nitrogen atmosphere, add tris(dibenzylideneacetone) dipalladium (0.27g, 0.3mmol), 70°C The reaction time is 1-4h, the liquid phase monitors the completion of the 4-bromo-9,9-dimethyl-9H-fluorene reaction, adding water to quench the reaction, separating the liquids, concentrating the organic phase, rinsing with petroleum ether through a silica gel column, and rinsing The washing solution was concentrated to obtain 9.02 g of N-(2-chlorophenyl)-9,9-dimethyl-9H-fluoren-4-amine as a colorless transparent oil, with a yield of 94% and a purity of 99.85%.
[0058] S2. In a 100mL three-necked flask, put the above-mentioned N-(2-chloro...
Embodiment 2
[0065] Embodiment 2: the synthesis of compound (4)
[0066] 1a. Synthesis of fluorenocarbazole:
[0067] Synthetic process is with the synthetic process of 1a in embodiment 1
[0068] 1b. Synthesis of 12-(3'-bromo-[1,1'-biphenyl]-3-yl)-fluorenocarbazole
[0069]
[0070] In Example 1, 4-bromo-4'-iodo-1,1'-biphenyl (3.59g, 10mmol) was replaced by 3-bromo-3'-iodo-1,1'-biphenyl (3.59g, 10mmol), other synthetic processes are the same as the synthetic process of 1b in Example 1 to obtain white powdery 12-(3'-bromo-[1,1'-biphenyl]-3-yl)-fluorenocarbazole 4.39g, yield 85%, purity 99.09%.
[0071] 1c. Synthesis of compound (4)
[0072] 5mmol) was replaced by 12-(3'-bromo-[1,1'-biphenyl]-3-yl)-fluorenocarbazole (2.57g, 5mmol), and other synthetic processes were the same as those of 1c in Example 1 , 2.75 g of compound (4) was obtained as a white powder, with a yield of 91% and a purity of 99.18%.
[0073] Mass spectrometer MALDI-TOF-MS (m / z) = 602.7805, theoretical molecular...
Embodiment 3
[0074] Embodiment 3: the synthesis of compound (6)
[0075] 1a. Synthesis of fluorenocarbazole:
[0076] Synthetic process is with the synthetic process of 1a in embodiment 1
[0077] 1b. Synthesis of 12-(3'-bromo-[1,1'-biphenyl]-4-yl)-fluorenylcarbazole
[0078]
[0079] In Example 1, 4-bromo-4'-iodo-1,1'-biphenyl (3.59g, 10mmol) was replaced by 3-bromo-4'-iodo-1,1'-biphenyl (3.59g, 10mmol), other synthetic processes are the same as the synthetic process of 1b in Example 1 to obtain white powdery 12-(3'-bromo-[1,1'-biphenyl]-4-yl)-fluorenocarbazole 4.33g, yield 84%, purity 99.10%.
[0080]
[0081] In Example 1, 12-(4'-bromo-[1,1'-biphenyl]-4-yl)-fluorenocarbazole (2.57g, 5mmol) was replaced by 12-(3'-bromo-[ 1,1'-biphenyl]-4-yl)-fluorenocarbazole (2.57g, 5mmol), diphenylamine (1.69g, 10mmol) was replaced by N-phenyl-[1,1'-biphenyl]- 4-Amine (2.45g, 10mmol), the other synthesis process was the same as that of 1c in Example 1 to obtain 2.95g of white powder compound (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com