A secondary antibody based on a portable blood glucose meter
A blood glucose meter and portable technology, applied in the field of medical/food testing, can solve problems such as restricting applications and detecting a single target, and achieve the effects of reducing laboratory costs, reducing use, and expanding application space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Construction of embodiment 1 expression strain
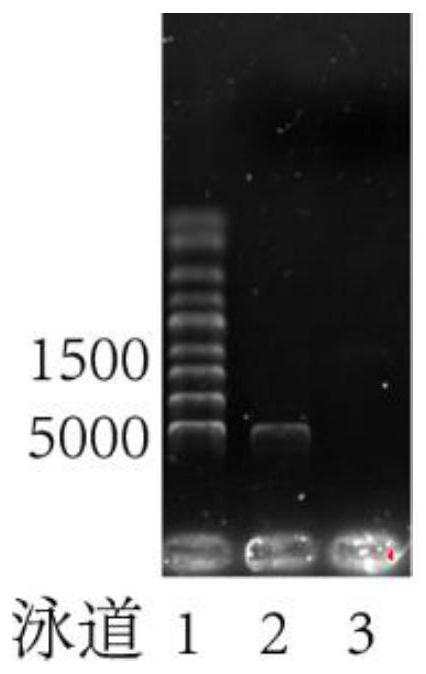
[0047] According to the base sequence (GeneID: 854644) of Saccharomyces cerevisiae sucrase Suc2 (amino acid sequence shown in SEQ ID NO.1), primers Suc2-F and Suc2-R were designed with Primer Premier 5.0, and the Saccharomyces cerevisiae genome was used as a template to amplify increase. The amplified fragments were recovered by a gel recovery kit. The recovered Suc2 gene fragment was double-digested with restriction endonucleases Nde I and Xho I and recovered ( figure 1 Swimming lane 3), and the commercialized vector pET22b ( figure 1 Swimming lane 2) for ligation, the product after ligation was transformed into commercialized Escherichia coli expression host BL21 (DE3), the transformed Escherichia coli was screened on a plate coated with ampicillin, and positive colonies were selected for sequencing verification, and the correct clone was verified as Expression strain of free sucrase Suc2.
[0048] In addition, in ord...
Embodiment 2
[0050] Fermentation and purification of the protein of embodiment 2
[0051] The fermentation method and purification method of Suc2, ZZ domain and the fusion protein Suc2-ZZ, ZZ-Suc2 adopt the conventional method and the conditions are the same, so the fermentation method of the fusion protein Suc2-ZZ is taken as an example to illustrate the fermentation and purification method of the protein in this example .
[0052] Take 20 μL of the fusion protein Suc2-ZZ glycerol bacteria to 3 mL of ampicillin-resistant LB medium (peptone 10 g / L, yeast powder 5 g / L, sodium chloride 10 g / L), culture overnight at 37 ° C, 200 rpm, and follow the steps of 4 % inoculum size was transferred to TB fermentation medium (peptone 12g / L, yeast powder 24g / L, glycerol 4g / L, KH 2 PO 4 23.1g / L, K 2 HPO 4 125.4g / L), until the bacterial concentration reaches OD 600 After the temperature is 0.6-1.2, the inducer IPTG with a final concentration of 0.4mM is added for induction, and the conditions for p...
Embodiment 3
[0055] Example 3 Evaluation of fusion protein sucrose hydrolysis ability
[0056] The enzyme reaction system is: 0.1M disodium hydrogen phosphate-citric acid buffer solution, pH 5.0, the final concentration of substrate sucrose is 1.5%, and the final concentration of pure enzyme is 10nM. The enzyme reaction conditions are: 40°C water bath for 5 minutes. After the reaction is complete, take 400 μL of the reaction solution and add it to a centrifuge tube pre-installed with 300 μL of DNS reagent, and place it in a boiling water bath for 5 minutes. After completion, immediately cool the test tube in ice water, and measure the absorbance with a spectrophotometer at a wavelength of 520 nm after appropriate dilution. The treatment method of the corresponding blank group is to first take 400 μL of enzyme-free reaction solution and add it to a centrifuge tube pre-installed with 300 μL of DNS reagent, and put it in a boiling water bath for 5 minutes. After appropriate dilution, the abs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com