Human targeting complement inhibitor protein mCR2-fH and application
A complement inhibitor and fusion protein technology, applied in the field of fusion proteins, can solve the problems that the inhibition effect is not as good as in vitro inhibition experiments, the protection rate of animal protection experiments is improved, etc., and achieve excellent anti-adhesion/anti-inflammatory targeted inhibition effect, crescent / Improvement of necrosis and improvement of inhibition efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1.CR2 mutant (mCR2)-fH fusion protein preparation
[0034] 1. Materials The expression vector was pEE14.1 (Lonza biologicals); CHO cells were used for protein expression, and the culture medium was DMEM containing 10% fetal bovine serum, which was purchased from Invitrogen. Mouse anti-fH mAb 1H4 and 1A10, mouse anti-human CR2mAb171, anti-goat erythrocyte IgM and all secondary antibodies were purchased from Sigma.
[0035] 2 methods
[0036] 2.1 The preparation of rabbit anti-CHO cell membrane and human fH antiserum was described in Harlow, E., and Lane, D. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory. Cold Spring Harbor, New York, USA. 1988: 726. method to obtain.
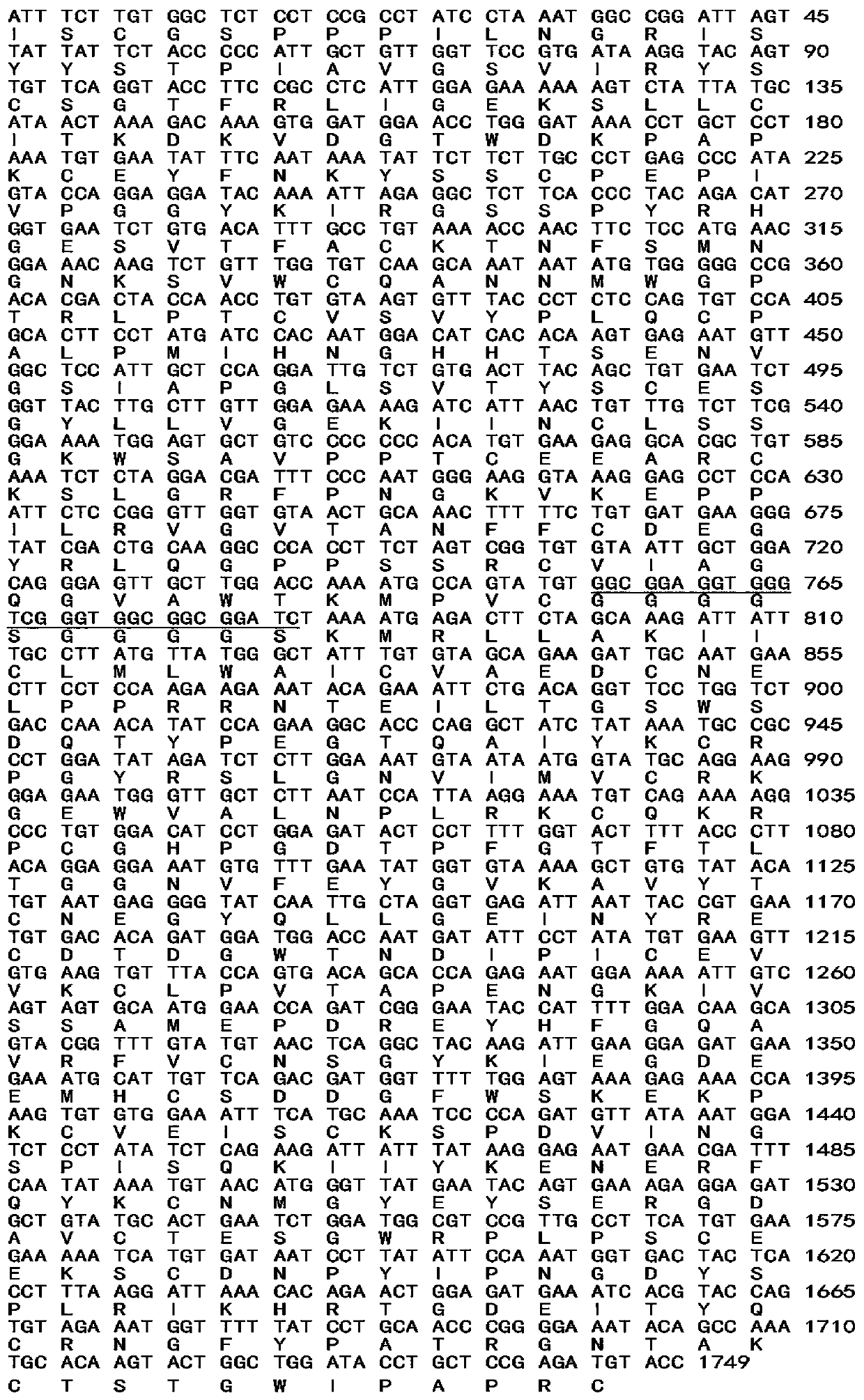
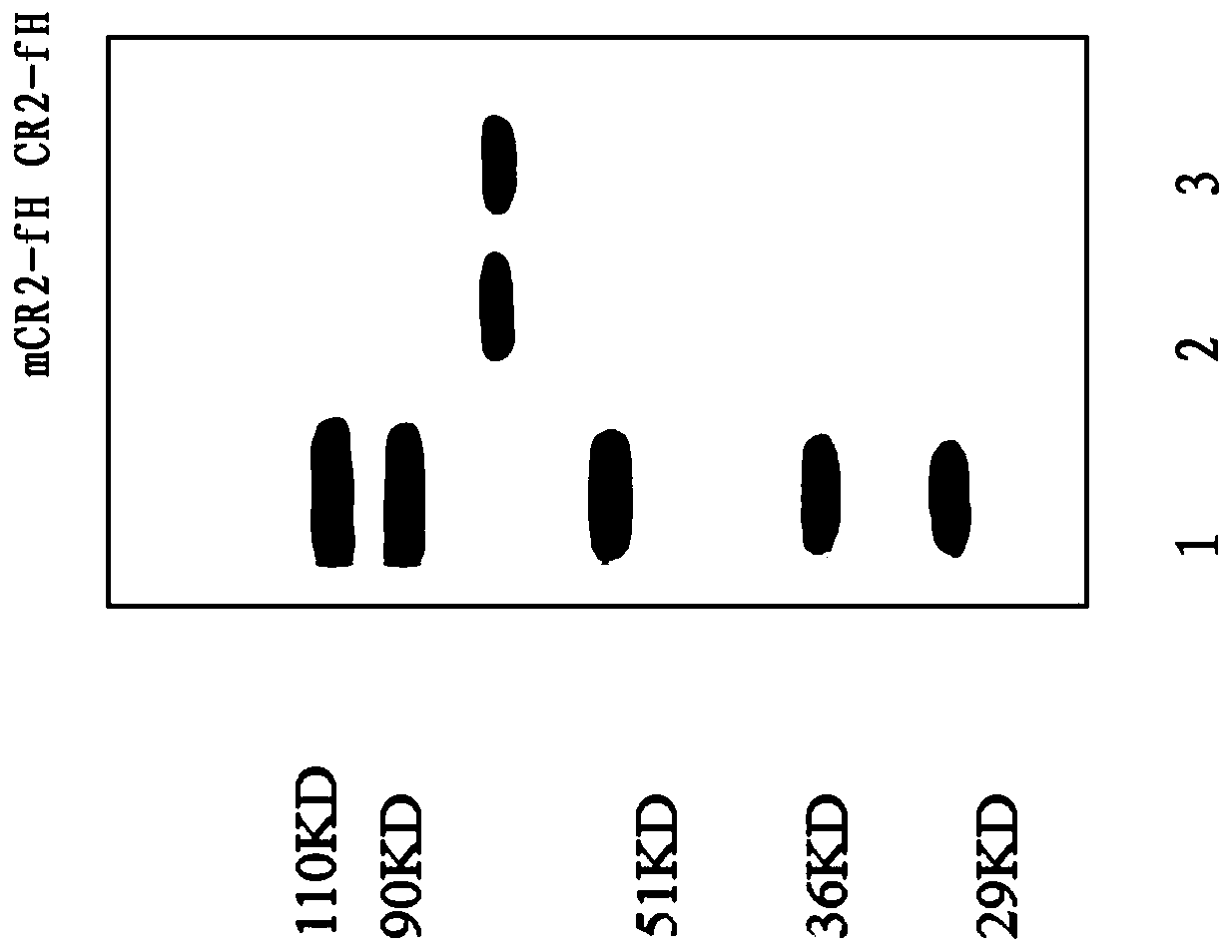
[0037] 2.2 Construction of expression recombinant and protein expression The cDNA structural gene is composed of 4 N-terminal SCR units encoding CR2 connected with the sequence encoding the extracellular region of fH. The complement inhibitor sequence is 322 amino acids encodi...
Embodiment 2
[0044] Example 2. Kinetic analysis of interaction between CR2 fusion protein and C3 ligand
[0045]Kinetic analysis of the interaction between CR2 fusion protein and biotin-labeled C3dg (C3dg-biotin) was detected by surface plasmon resonance (SPR) detection system (BIAcore3000 instrument). Human C3dg-biotin (Guthridge, J.M., et al. Structural studies solution of the recombinant N-terminal pair of short consensus / complement repeat domains of complement receptor type 2 (CR2 / CD21) and interactions with its ligand C3dg.Biochemistry.2001,40(20):5931–5941.) were injected onto the BIAcore streptavidin sensor chip at a speed of 2μL / min for 20min, and the buffer was 0.5× PBS (pH7.4) (containing 0.5g / L Tween20). SPR signals acquired from captured C3dg yielded BIAcore response units (range 250 to 500). The group without fusion protein was used as the control. After washing with 0.5×PBST (0.5g / LTween20) at a flow rate of 25μL / min at 25°C, the affinity of the CR2 fusion protein was eva...
Embodiment 3
[0049] Embodiment 3. Complement lysis experiment
[0050] To measure the inhibitory activity on complement, 60%-80% confluent CHO cells were separated with ethylenediaminetetraacetic acid, washed twice with DMEM, and then resuspended in DMEM to make the final concentration of 10 6 cells / mL. Add 100mL / L rabbit anti-CHO cell membrane antiserum to the cell suspension and react at 4°C for 30min to sensitize the cells. Then the antiserum was discarded, and the cells were resuspended in NHS diluted with DMEM to a final volume of 50 μL or 100 μL. After 60 minutes at 37°C, the cell viability was measured by placenta blue staining and exclusion method (both live and dead cells were counted). The recombinant fusion protein was diluted with DEME and added to NHS first, and then added to CHO cell suspension. The final concentration was based on the control CHO cells lysed by 100 g / L NHS which can cause about 90% antibody sensitization. Complement-mediated inhibition of erythrocyte lys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com