Recombinant mIL-22BP vector, and liposome complex and preparation methods and application thereof
A technology of IL-22BP, liposome complex, applied in the directions of botanical equipment and methods, biochemical equipment and methods, liposome delivery, etc., can solve the problem of liposomes with no mouse IL-22BP gene. Compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Construction of mIL-22BP gene expression plasmid
[0042] 1. Acquisition of mIL-22BP gene
[0043] 1) According to the coding sequence of the mIL-22BP gene (Gene ID: 237310, NM_178258) in the NCBI (National Center for Biotechnology Information) database (sequence shown in SEQ ID NO.1), synthesize a full-length mIL cDNA plasmid of -22BP gene sequence.
[0044] 2) Design and synthesize corresponding primers
[0045] Upstream primer: 5'-GGGAAAGCTTATGATGCCTAAGCATTGCCTTTCTAGG-3', (SEQ ID NO.4)
[0046] The design introduces a Hind III restriction site;
[0047] Downstream primer 5'-GGGATCTAGATCATGGAATGTGCACACATCTCTCC-3', (SEQ ID NO.5)
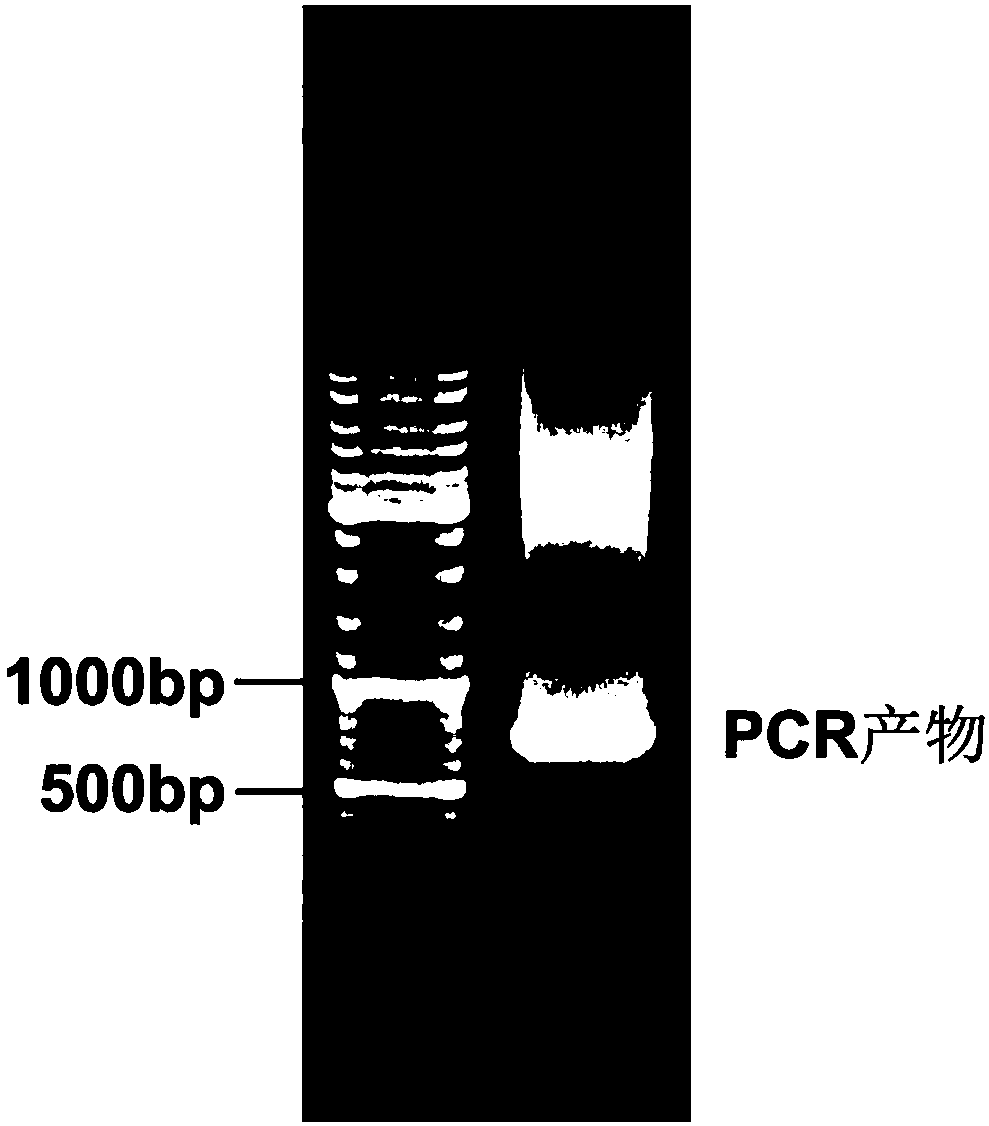
[0048] The Xbal I restriction site was designed and introduced, the DNA fragment of mIL-22BP was obtained by PCR amplification, and the fragment was recovered by agarose gel electrophoresis to obtain the mIL-22BP gene fragment.
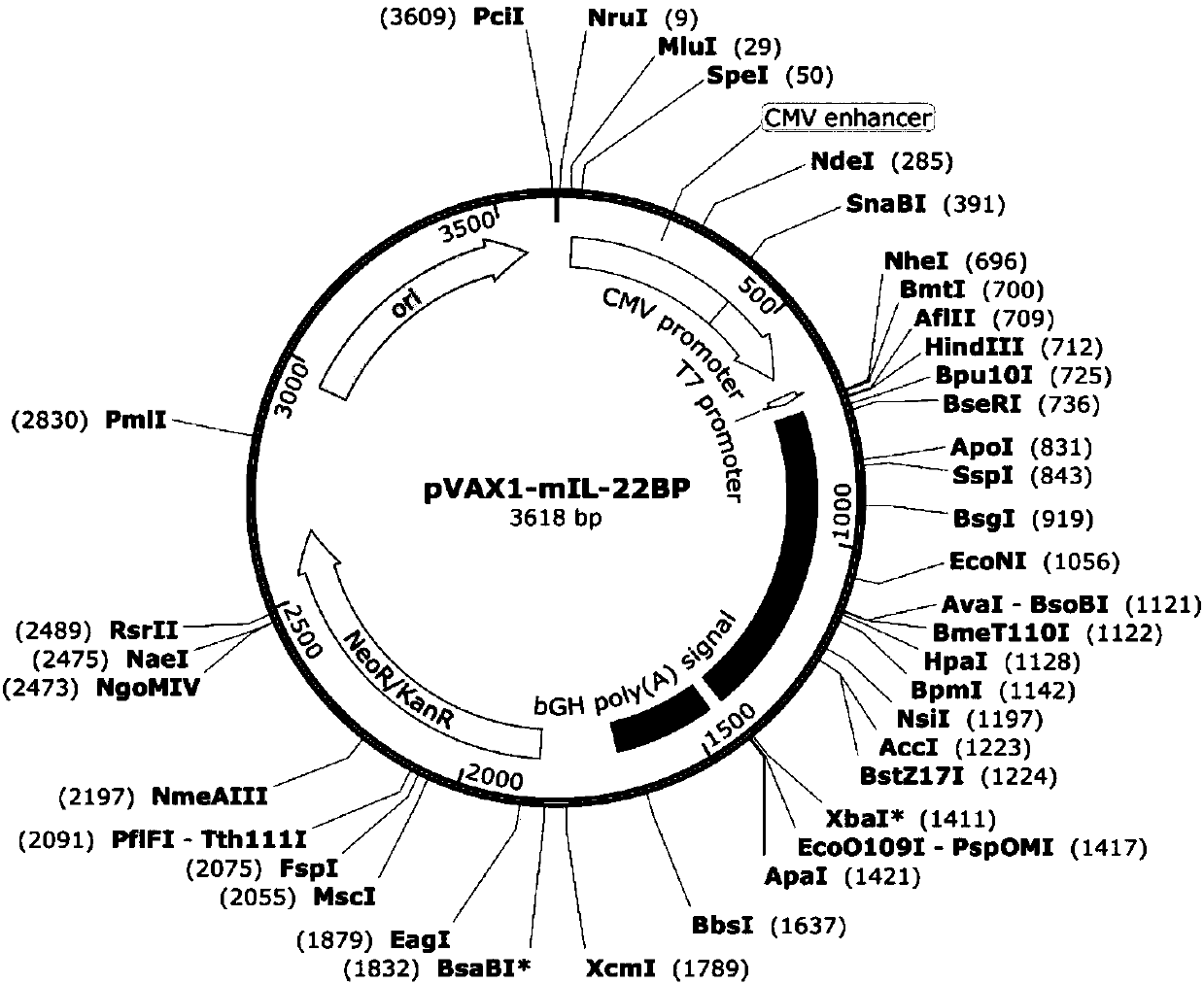
[0049] 2. Cloning construction of pVAX1-mIL-22BP plasmid
[0050] 1) Use Hind III and Xbal I restr...
Embodiment 2
[0052] Example 2 Preparation of recombinant mIL-22BP carrier-liposome complex
[0053] 1. Preparation of DOTAP-Chol cationic liposomes
[0054] The cationic lipid DOTAP and cholesterol (Chol) were mixed at a molar ratio of 1:1, and the mixture was dissolved in chloroform, and placed on a rotary evaporator at 60°C for 120 minutes to form a film. The formed film was taken out and dissolved in 5% glucose solution, then shaken in a water bath at 60°C for 20 minutes, and the mixture was ultrasonically disrupted at 0°C at 400w for 10 minutes to obtain a 5 mg / ml cationic liposome suspension.
[0055] 2. Preparation of mIL-22BP gene expression plasmid
[0056] The pVAX1-mIL-22BP plasmid was transformed into DH5α E. coli competent cells and spread on LB plates containing kanamycin resistance. After 24 hours, the clones were picked and placed in 3 mL of LB medium containing kanamycin for shaking overnight, and the pVAX1-mIL-22BP plasmid recombinant plasmid was extracted the next day. ...
Embodiment 3
[0059] Example 3 Recombinant mIL-22BP carrier liposome complex anti-tumor test
[0060] In order to study the anti-tumor effect of the pVAX1-mIL-22BP plasmid cationic liposome complex in vivo, a mouse model of peritoneal metastases of colon cancer was established in the peritoneal cavity of Balb / C mice (6-8 weeks old, female). Specifically, the CT26 mouse colon cancer cells cultured in vitro were digested with trypsin, and the volume was fixed in 1640 medium without serum and antibiotics, and 2.5×10 5 cells, after 3 days of cell inoculation, began to carry out random grouping treatment (every group of 5) as follows;
[0061] A) blank control group: 5% glucose solution;
[0062] B) simple cationic liposome group;
[0063] C) pVAX1-mIL-22BP treatment group: the cationic liposome / pVAX1-mIL-22BP complex was placed in 5% glucose solution.
[0064] Treat by intraperitoneal injection, the plasmid DNA cationic liposome complex ratio is as follows: plasmid DNA (5 μ g): cationic lipo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com