A kind of saddle-shaped cyclic compound containing thiadiazo aromatic aldehyde schiff base and preparation method thereof
A technology for cyclic compounds and aromatic aldehydes, which is applied in the field of thiadiazonium-containing aromatic aldehyde Schiff base saddle-type cyclic compounds and their preparation, can solve the problems of inability to satisfy scientific research and actual production, and achieves convenient industrial scale production, The effect of mild reaction temperature and cheap and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The second aspect of the present invention provides the preparation method of the Schiff base saddle-type cyclic compound containing thiadiazo aromatic aldehyde, comprising:
[0040] Under nitrogen atmosphere, mix 2-amino-5-mercapto-1,3,4-thiadiazole and p-fluorobenzaldehyde into ethanol, add trifluoroacetic acid, heat to reflux, and purify to obtain the product.
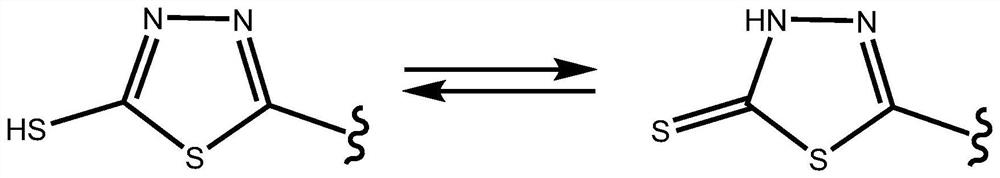
[0041] In the present invention, using ethanol solvent as the reaction medium, trifluoroacetic acid can protonate the N ion on the thiadiazide ring, and promote the "head-to-tail" addition between Schiff base molecules to obtain a cyclic compound.
[0042] In another specific embodiment of the present invention, the molar ratio of 2-amino-5-mercapto-1,3,4-thiadiazole, p-fluorobenzaldehyde and trifluoroacetic acid is 1:1~2:0.05~ 0.15; By controlling the ratio of the amount of each reactant, it is beneficial to improve the reaction yield and save the reaction time;
[0043] In yet another specific embodiment o...
Embodiment 1
[0050] Under nitrogen range, add 15 milliliters of ethanol to 25 milliliters of two-necked flasks, 396mg (3mmol) 2-amino-5-mercapto-1,3,4-thiadiazole and 409.2mg p-fluorobenzaldehyde are mixed in ethanol, drop into 0.02ml of trifluoroacetic acid was refluxed at 80°C for 2 hours, the solution changed from light yellow to orange yellow to light yellow precipitate, filtered, washed with 20 ml of water three times, and then washed with ether. The washing solvent was evaporated, and recrystallized overnight using dichloromethane / petroleum ether (v / v=1:1.5), to finally obtain 451 mg of light yellow crystalline powder with a yield of 81%.
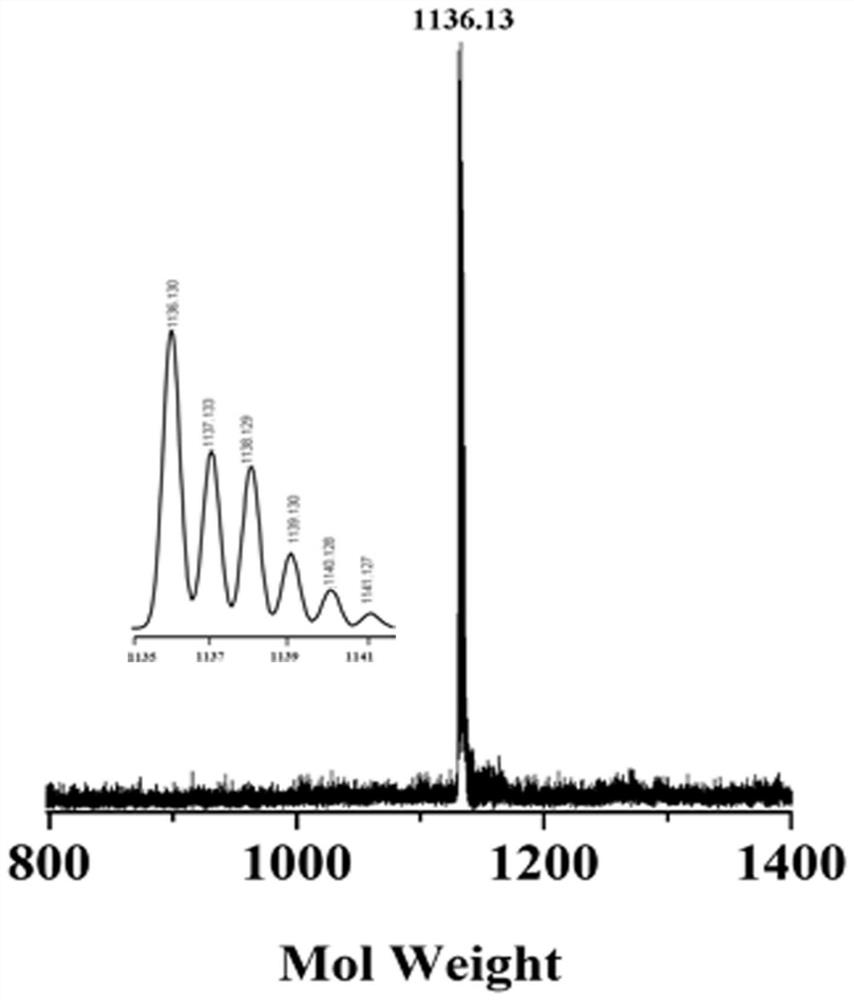
[0051] Mass Spectrum: (calculated value) for C 44 h 44 f 4 N 12 o 4 S 8 : 1136.30 (M+); (measured value): 1136.13. Elemental analysis: (calculated value) (%) for C 44 h 44 f 4 N 12 o 4 S 8 : C, 46.46; H, 3.90; N, 14.78. Found: C, 46.24; H, 3.81; N, 14.22.
Embodiment 2
[0053] Under nitrogen range, 10 ml of ethanol was added to a 25 ml two-necked flask, 396 mg (3 mmol) of 2-amino-5-mercapto-1,3,4-thiadiazole and 500 mg of p-fluorobenzaldehyde were mixed into ethanol, and 0.01 ml of trifluoroacetic acid, refluxed at 80°C for 2 hours, the solution changed from light yellow to orange yellow to light yellow precipitate, filtered, washed three times with 20 ml of water, and then washed with ether. The washing solvent was evaporated, and dichloromethane / petroleum ether (v / v=1:1.5) was used for recrystallization overnight to finally obtain 462 mg of light yellow crystalline powder with a yield of 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com