Method for constructing tissue engineered cartilage by using bone-marrow mesenchymal stem cells
A bone marrow mesenchymal and tissue engineering technology, applied in the direction of bone/connective tissue cells, animal cells, vertebrate cells, etc., can solve the problems of bone marrow mesenchymal stem cells new tissue engineering cartilage vacuolization and other problems, and achieve central vacuolation. The effect of chemical suppression and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
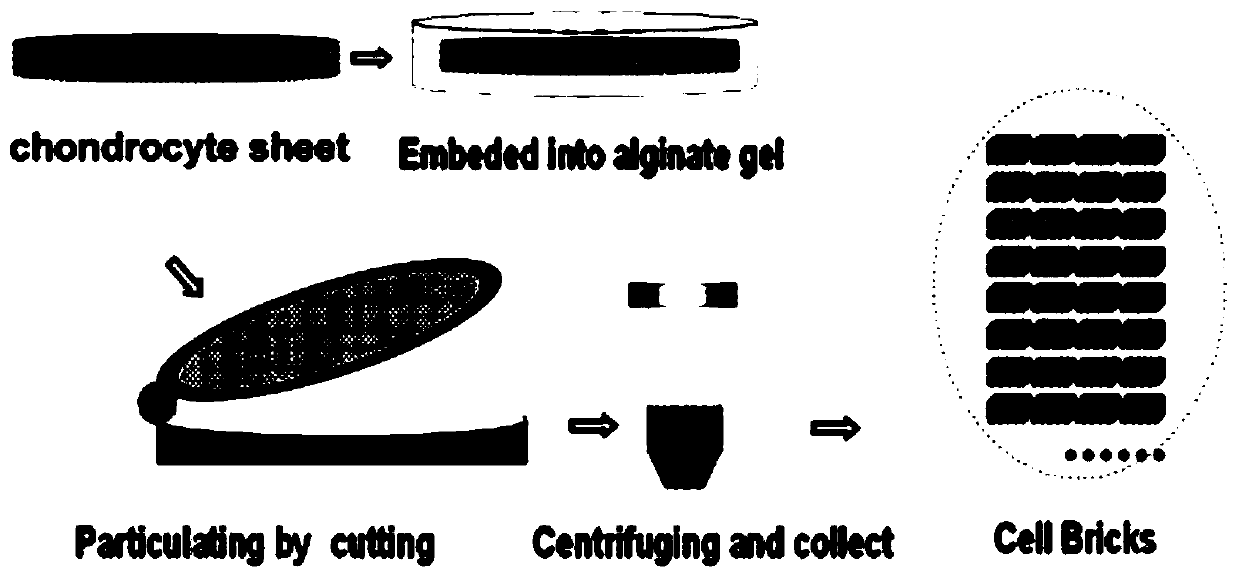
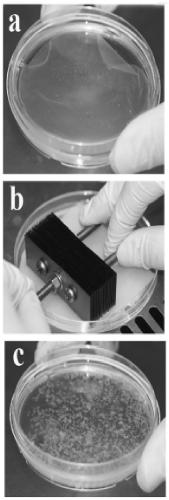
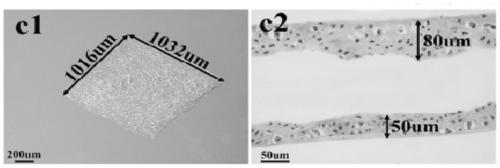
[0025] Example 1. Preparation of chondrocyte aggregates (cell bricks)
[0026] Take the ear cartilage of 1-month-old New Zealand white rabbit, cut it into pieces, digest it with 0.2% (wt) type II collagenase (Gibco, USA) solution at 37°C for 12 hours, and then take the single cell suspension in a 15mL centrifuge tube Centrifuge at 1000rpm for 5min. The supernatant was discarded, washed once with PBS, centrifuged at 1000 rpm for 5 min, and the cells were resuspended in membrane-forming inducing solution. Take 6.5×10 5 cells / cm 2 The concentrations were grown in 6-well plates. Afterwards, the film-forming inducing medium was used to continue culturing, and the medium was changed every 3 days. The film-forming induction medium used was high-glucose DMEM medium (Hyclone, U.S.), containing 20% fetal bovine serum (Gibco, U.S.), 272 μg / mL glutamine (Amresco, U.S.), 50 μg / mL ascorbic acid (Amresco, U.S. ), 50 μg / mL penicillin (Amresco, USA), 30 μg / mL penicillin (Amresco, USA)...
Embodiment 2
[0029] Example 2. Preparation of platelet-rich plasma (PRP)
[0030] Take 2-month-old New Zealand white rabbit ear vein blood, add 3.8% (wt) sodium citrate solution anticoagulant according to 10% of the total volume, and prepare PRP by two-step centrifugation: whole blood is centrifuged at 1800rpm at room temperature After 8 minutes, divide into upper, middle and lower layers, the upper layer is platelet-poor plasma, the middle layer is platelet-rich plasma, and the lower layer is red blood cells; transfer the upper and middle layers of plasma to another centrifuge tube, centrifuge at 3600rpm for 8 minutes, discard the upper layer 3 / 4 of the platelet-poor plasma, and the rest of the platelets were blown repeatedly with a dropper to form platelet-rich plasma, in which the final concentration of platelets was 20.9±1.1×10 8 cells / mL and kept on ice for future use.
Embodiment 3
[0031] Example 3. Preparation, in vivo growth and detection results of BMSC-cell brick-platelet cell complexes
[0032] Experimental operation:
[0033] Suspend the BMSC subcultured to the P3 generation and the cell brick (CB) prepared in Example 1 in 500 μL of the platelet-rich plasma prepared in Example 2, mix well, and add 50 μL of thrombin solution containing 100 mg / mL calcium chloride to it . Then injected subcutaneously into nude mice for in vivo growth. In this experiment, we focused on the effect of the ratio between the number of BMSCs and the number of chondrocytes contained in cell bricks on the formation of nascent tissue-engineered cartilage. The sum of the number of BMSCs and chondrocytes used was 3 × 10 8 indivual. The experiments performed were divided into three groups, including ratios between BMSCs and chondrocytes of 1:2, 1:1, and 2:1, hereinafter denoted as B1CB2, B1CB1, and B2BC1, respectively. After 8 weeks of in vivo growth, the samples were take...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com