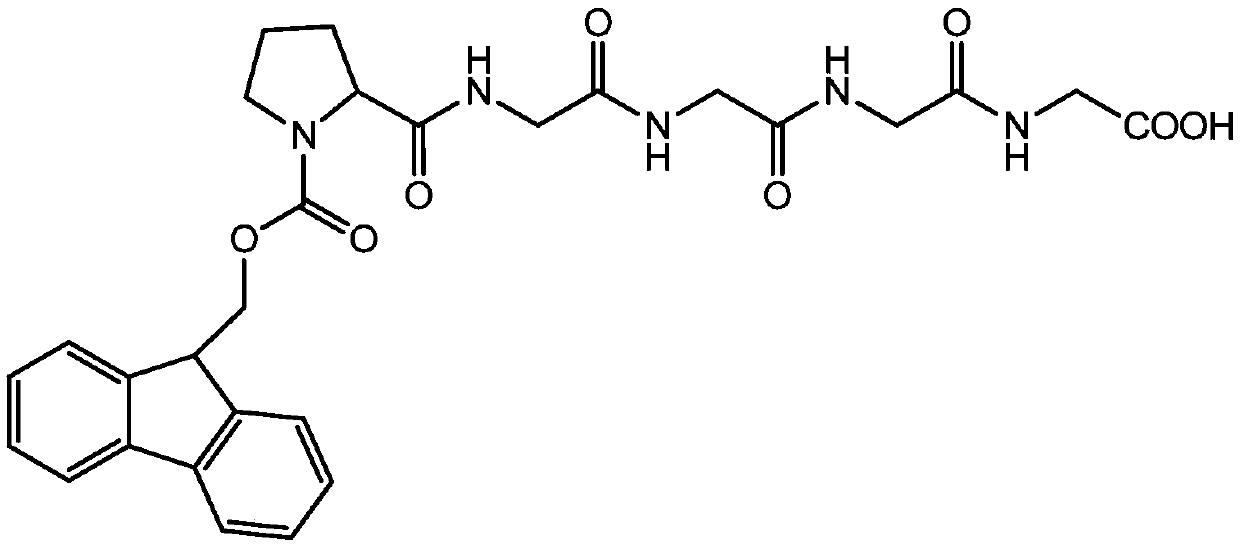
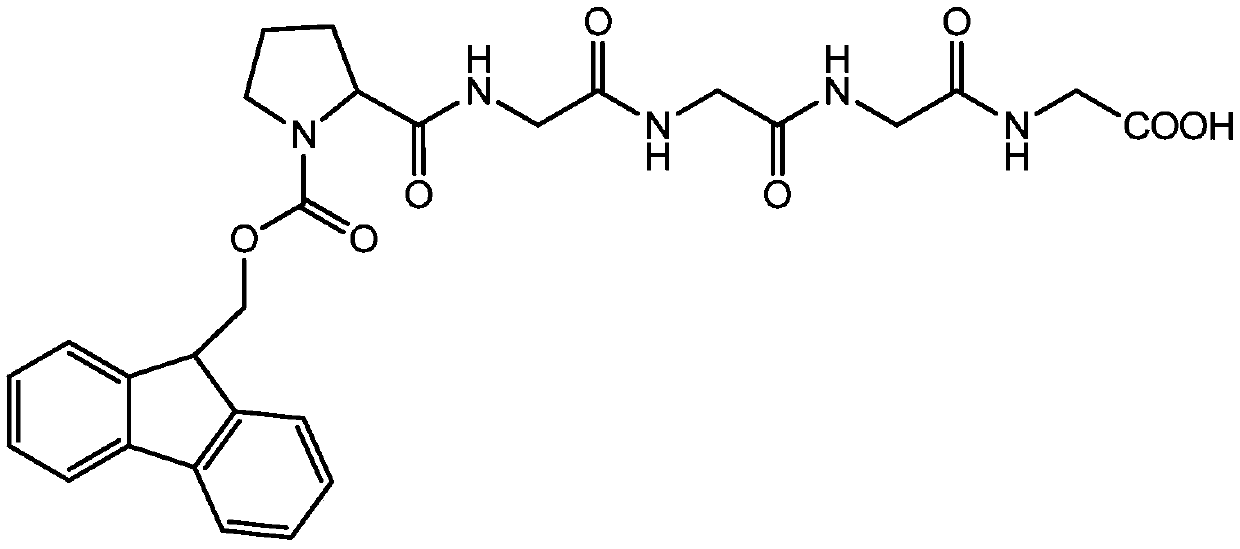
Technical method for synthesizing bivalirudin protected pentapeptide fragment by mass high-performance liquid chromatography method
A technology of high-efficiency liquid phase and process method, which is applied in the field of high-volume high-efficiency liquid phase synthesis of bivalirudin protected pentapeptide fragments, which can solve the problems of difficult purification, easy production of impurities, and low yield, and achieve controllable impurities , less environmental pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A process method for synthesizing bivalirudin-protected pentapeptide fragments by high-efficiency liquid phase method in large batches, the specific steps are as follows:
[0046] (1) Synthesis of tripeptide Fmoc-Pro-2Gly-OH
[0047] Fmoc-Pro-OSu+H-Gly-Gly-OH(Na 2 CO 3 )→Fmoc-Pro-2Gly-OH
[0048] Add sodium carbonate (42.4g, 0.4mol) to 300 ml of purified water, stir to dissolve, add bisglycine peptide (27g, 0.2mol) and stir to dissolve, after stirring to dissolve, add Fmoc-Pro-OSu (87g) dropwise , 0.2mol) solution of dioxane (300 ml), dripping is completed, after 6 hours of reaction, spot plate detection Fmoc-Pro-OSu reaction is complete. Add 1200 mL of purified water to the reaction solution, stir evenly, adjust the pH of the reaction solution between 2-3 with 3M hydrochloric acid solution, stand for crystallization, suction filtration, wash with purified water until the product is neutral, and dry to obtain the tripeptide Fmoc-Pro- 2Gly-OH 75.0 g (0.17 mol), purity ...
Embodiment 2
[0055] A process method for synthesizing bivalirudin-protected pentapeptide fragments by high-efficiency liquid phase method in large batches, the specific steps are as follows:
[0056] (1) Synthesis of tripeptide Fmoc-Pro-2Gly-OH
[0057] Fmoc-Pro-OSu+H-Gly-Gly-OH(NaHCO 3 )→Fmoc-Pro-2Gly-OH
[0058] Add sodium bicarbonate (33.6g, 0.4mol) to 300 ml of purified water, stir to dissolve, add glycine peptide (27g, 0.2mol) and stir to dissolve, after stirring to dissolve, add Fmoc-Pro-OSu ( 87g, 0.2mol) solution of dioxane (150ml), dripping is completed, after 8 hours of reaction, spot plate to detect that the reaction of Fmoc-Pro-OSu is complete. Add 1200 mL of purified water to the reaction solution, stir evenly, adjust the pH of the reaction solution between 2-3 with 3M hydrochloric acid solution, stand for crystallization, suction filtration, wash with purified water until the product is neutral, and dry to obtain the tripeptide Fmoc-Pro- 2Gly-OH 60.1 g (0.136 mol), purity ...
Embodiment 3
[0065] A process method for synthesizing bivalirudin-protected pentapeptide fragments by high-efficiency liquid phase method in large batches, the specific steps are as follows:
[0066] (1) Synthesis of tripeptide Fmoc-Pro-2Gly-OH
[0067] Fmoc-Pro-OSu+H-Gly-Gly-OH(NaHCO 3 )→Fmoc-Pro-2Gly-OH
[0068] Add sodium carbonate (42.4g, 0.4mol) to 300 ml of purified water, stir to dissolve, add bisglycine peptide (27g, 0.2mol) and stir to dissolve, after stirring to dissolve, add Fmoc-Pro-OSu (87g) dropwise , 0.2mol) methanol (300 ml) solution, dripping is completed, after 8 hours of reaction, spot plate detection Fmoc-Pro-OSu reaction is complete. Add 1200 mL of purified water to the reaction solution, stir evenly, adjust the pH of the reaction solution between 2-3 with 3M hydrochloric acid solution, stand for crystallization, suction filtration, wash with purified water until the product is neutral, and dry to obtain the tripeptide Fmoc-Pro- 2Gly-OH 55.8g (0.126mol), purity 97.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com