Degradable polymer based on ring-opening polymerization of valerolactone derivatives and its preparation method and use
A technology of degrading polymers and ring-opening polymerization, which can be applied to other methods of inserting foreign genetic materials, recombinant DNA technology, etc., can solve the problems of large differences in transfection efficiency, unfavorable modification, and single structure of polyester carriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A degradable polymer based on ring-opening polymerization of valerolactone derivatives, the structural formula (I) of the compound is as follows:
[0049]
[0050] In formula (I), R 1 Is a substituted or unsubstituted macrocyclic polyamine [12]aneN 3 group, R 2 is a sulfur-containing substituent, R 3 is a substituted or unsubstituted phenyl group, m and n are positive integers greater than or equal to 10.
[0051] Wherein, when the degradable polymer is represented by a compound of formula (I-1):
[0052]
[0053] Among them, R 1 for R 2 for The polymer with n=m=10 is designated as polymer B1a; R 1 for R 2 for n=20, m=10 is recorded as polymer B1b.
[0054] When the degradable polymer is represented by a compound of formula (I-2):
[0055]
[0056] Among them, R 1 for R 2 for The polymer with n=20 and m=10 is designated as polymer B2a.
[0057] When the degradable polymer is represented by a compound of formula (I-3):
[0058]
[005...
Embodiment 2
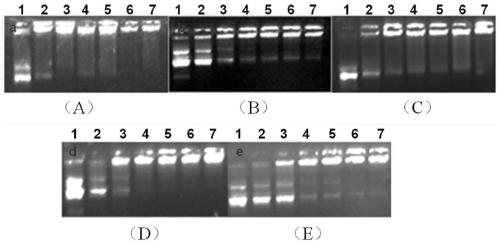
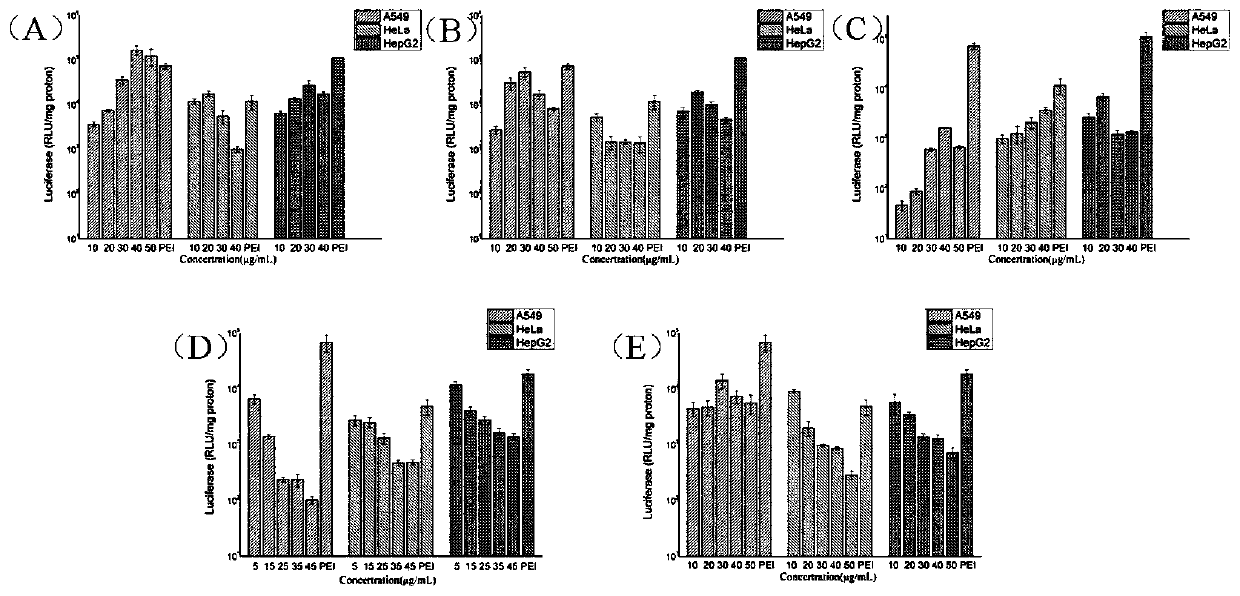
[0073] Prepare solutions of different concentrations of B1a / DOPE, B1b / DOPE, B2a / DOPE, B3a / DOPE and B3b / DOPE (1:2, molar ratio) respectively, and form a complex with pGL-3 plasmid DNA at 37°C Incubate for 0.5 h, and then add it into different gel wells to conduct DNA agarose gel retardation experiments to obtain the aggregation of DNA by different concentrations of polymers.
[0074] figure 1 A~1E are the agarose gel retardation experiment results of the pGL-3DNA of polymer B1a / DOPE of the present invention, B1b / DOPE, B2a / DOPE, B3a / DOPE and B3b / DOPE respectively; figure 1 It can be concluded that the polymer formed based on valerolactone ring-opening polymerization of the present invention can effectively condense DNA to form nanoparticles, and can be used as a non-viral gene carrier.
Embodiment 3
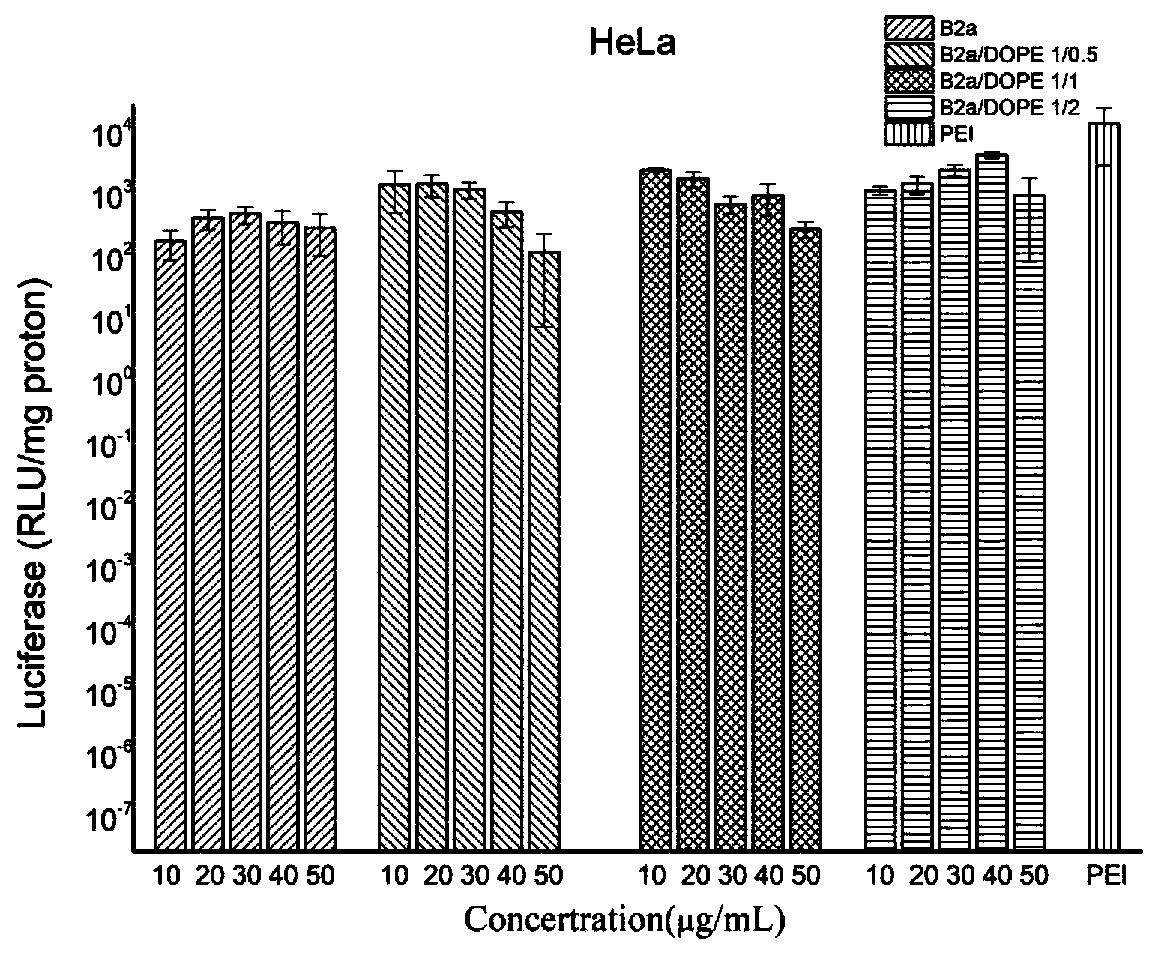
[0076] Polymer B2a and its cationic liposomes formed with DOPE at 1:0.5, 1:1, 1:2 (molar ratio) and PGL-3DNA were incubated at 37°C for 30 minutes before administration, and the cultured HeLa was added In the cells, act for 5 hours, suck out the compound, and then replace the cell culture medium with fresh DMEM culture medium containing 10% FBS and culture for 48 hours. After removing the medium, add 120 μL of cell lysate, lyse the cells, measure the luminescence intensity and protein content, take PEI 25k as the standard, and the luminescence intensity per milligram of protein (RLU / mg protein) indicates the transfection efficiency of compound B2a .
[0077] figure 2 It is the luciferase expression result of the compound B2a of the present invention and its liposome formed with DOPE as a non-viral gene carrier in HeLa cells at different concentrations and different ratios of B2a / DOPE; PEI 25k is used as a reference.
[0078] figure 2 The middle X-axis is B2a and its diffe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com