Preparation method of ticagrelor medicinal crystal form II
A technology of ticagrelor and crystal form, applied in the field of preparation of ticagrelor, can solve the problems of high toxicity of the crystallization solvent chloroform, unsuitable for large-scale production, uneven crystal particles, etc., and achieves small particle size and high purity. , the effect of simple process preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In a 1L reaction flask, sequentially add toluene (300ml), 2-(3AR,4S,6R,6AS)-6-(5-amino-6-chloro-2-propylthio-4-pyrimidinyl)amino tetra Hydrogen-2,2-dimethyl-4H-cyclopenta-1,3-dioxolan-4-yl]oxy]-ethanol (Intermediate 1) (62.6 g, 149 mmol), acetic acid ( 50.0g, 834mmol), stirring and cooling down to 0-10°C, and adding sodium nitrite aqueous solution (10.8g sodium nitrite / 25ml water) dropwise. After dropping, the temperature was raised to 20-30°C, stirred for 10 minutes, and an aqueous solution of potassium carbonate (61.8g potassium carbonate / 125ml water) was added dropwise to adjust the pH to 7-8.
[0033] In a 3L reaction bottle, add the organic phase of the previous step, stir and cool down to 0-10°C, add the pre-cooled concentrated hydrochloric acid and methanol mixture (271.9g concentrated hydrochloric acid / 250ml methanol) dropwise, stir for 5 minutes, and let stand to separate the liquid . Add 1L of purified water and 0.5L of ethyl acetate to the aqueous phase, co...
Embodiment 2
[0037] The preparation method of the organic phase solution of ticagrelor is the same as that in Example 1.
[0038] Add n-heptane dropwise to the organic phase in a 2L reaction bottle. After dropping, drop to 0-10°C, stir for 48h, centrifuge, and vacuum-dry the filter cake at 40-45°C to constant weight (differential weight is less than 0.5%) to obtain White solid, yield range: 60-65%.
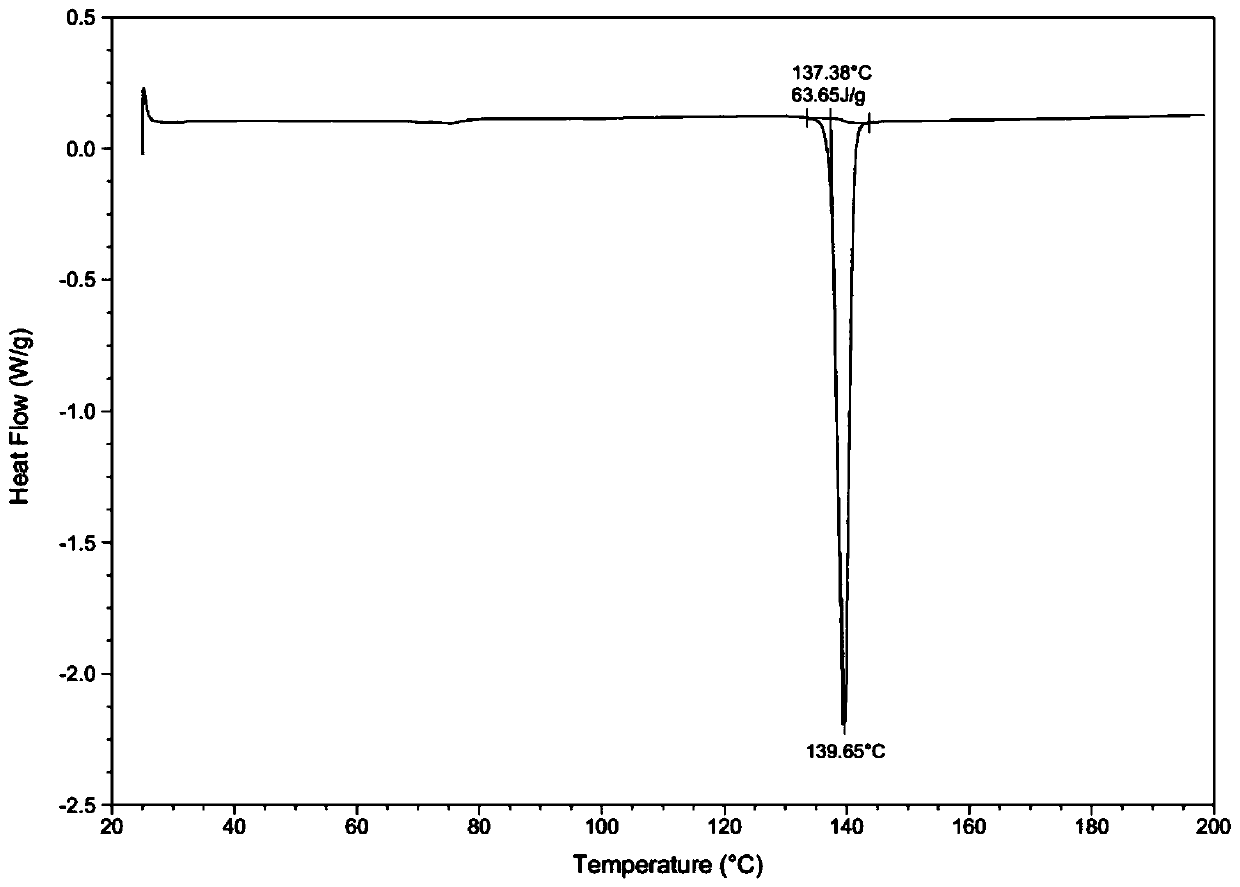
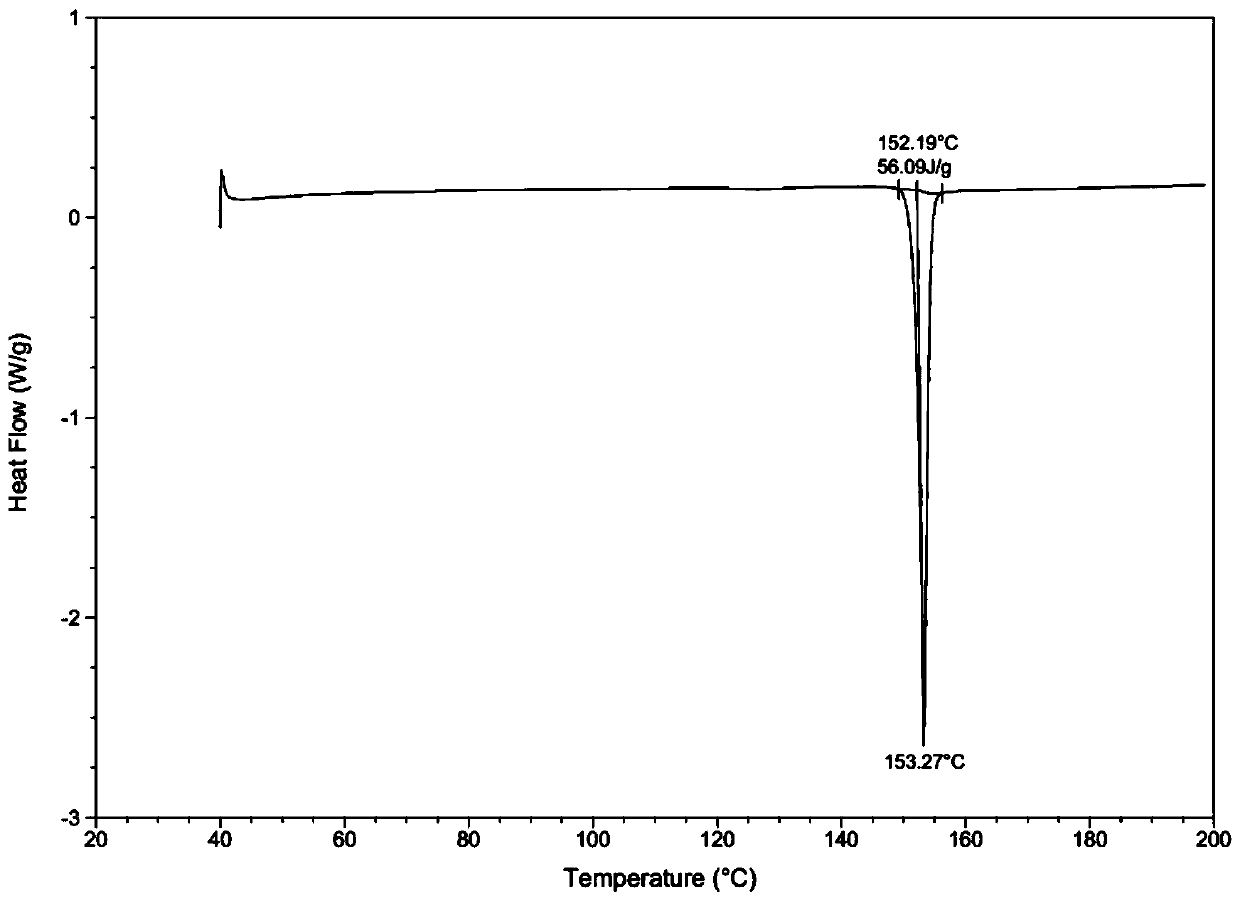
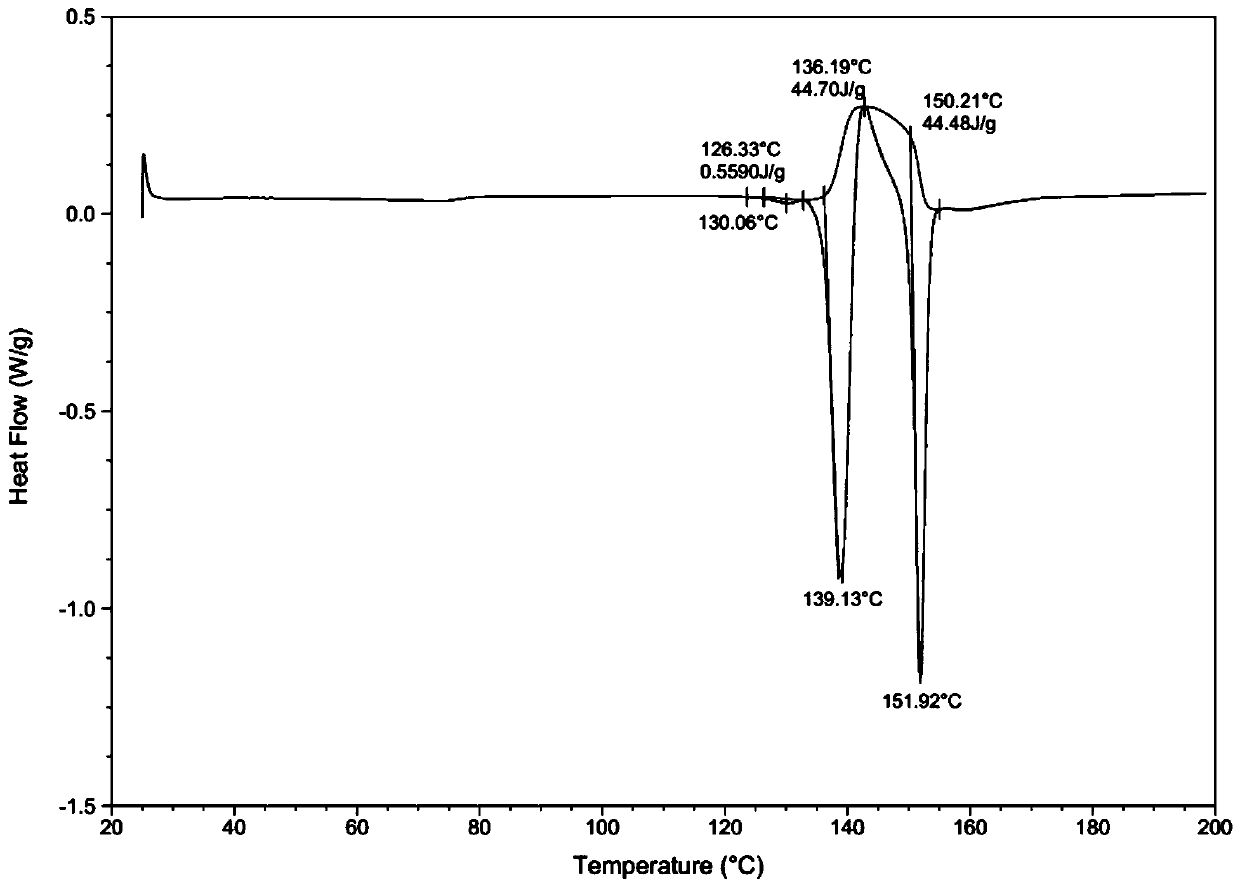
[0039] Through HPLC detection, the product purity is 99.2%; DSC and XRD detection, the sample is II crystal form; Malvern particle size analyzer detection particle size range is less than 20um.
Embodiment 3
[0041] The preparation method of the organic phase solution of ticagrelor is the same as that in Example 1.
[0042] Concentrate the organic phase in a 2L reaction bottle at 40-45°C to the remaining about 0.5L, raise the temperature of the residue to 60-70°C, add cyclohexane dropwise under temperature control, after the drop is completed, lower it to 0-10°C, stir for 3 hours, and centrifuge , the filter cake was vacuum-dried at 40-45° C. to constant weight (the difference in weight was less than 0.5%) to obtain an off-white solid with a yield range of 75-80%.
[0043] Through HPLC detection, the product purity is 99.5%; DSC and XRD detection, the sample is II crystal form; Malvern particle size analyzer detection particle size range is 30-40um.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com