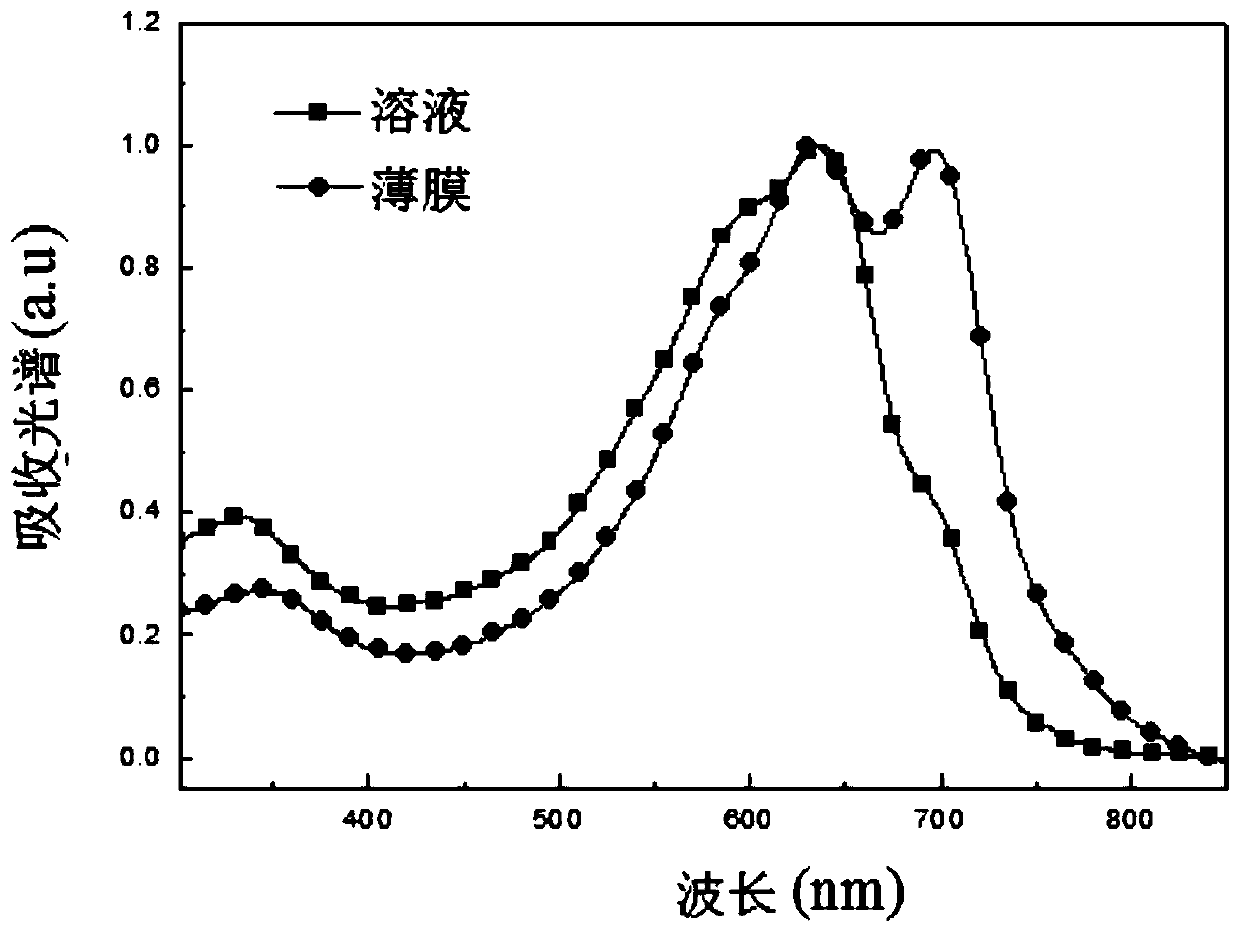
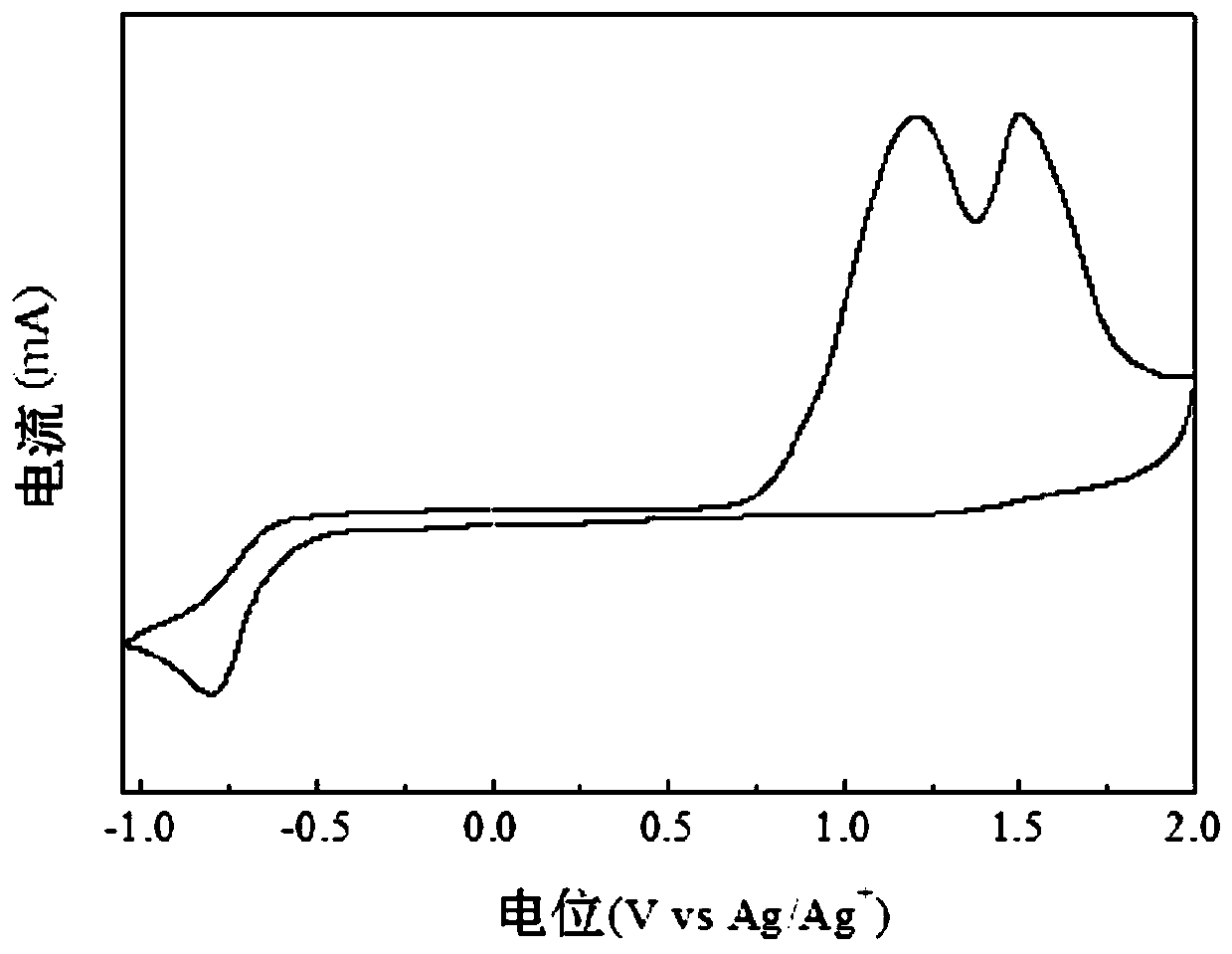
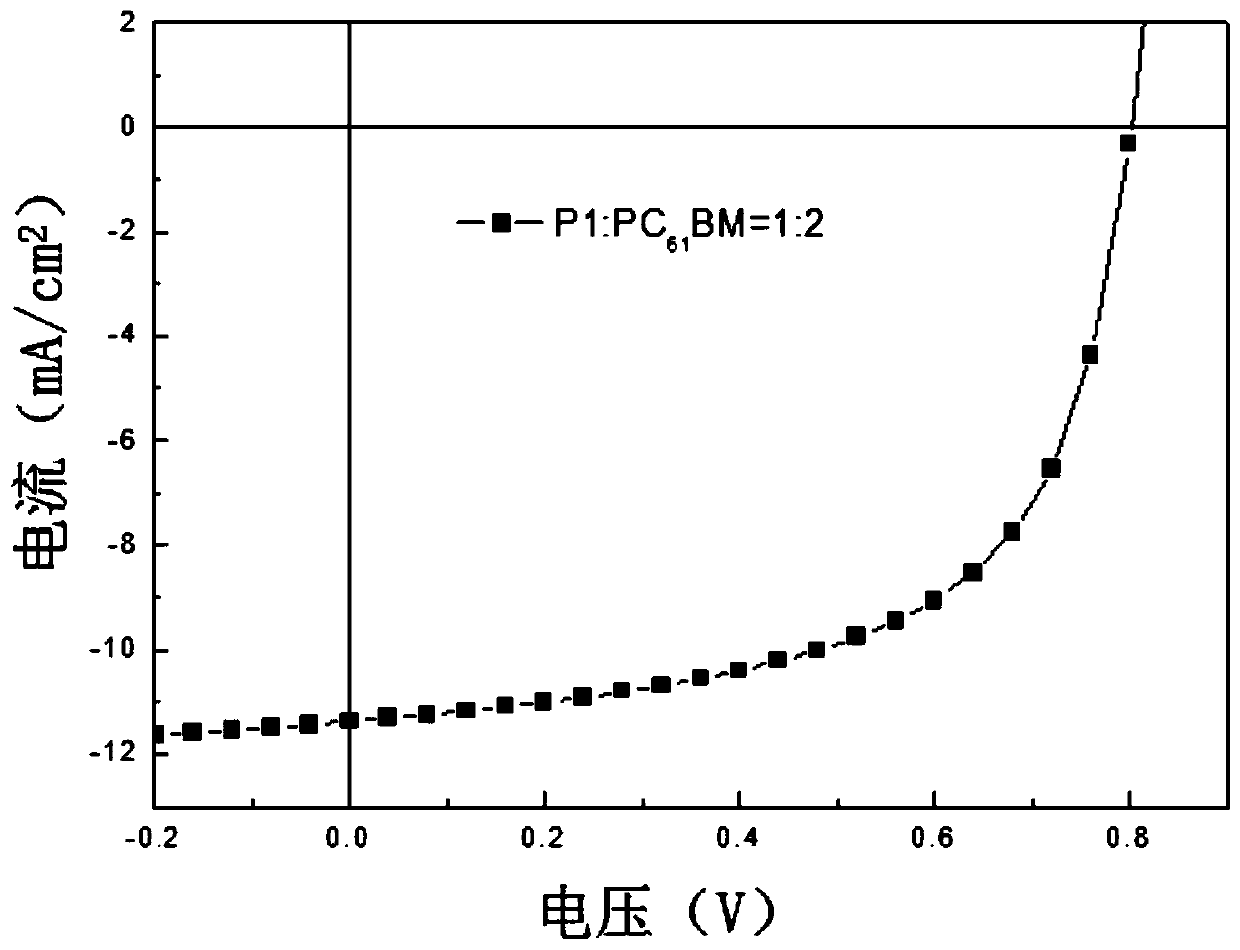
Two-dimensional conjugated benzodithiophene and furan and pyrazine copolymer, preparation method and application thereof
A benzodithiophene, two-dimensional conjugation technology, used in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problems of limited application, insufficient photoelectric conversion efficiency, etc., and achieve a wide absorption spectrum and good photoelectric conversion. The effect of efficiency, good machinability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 4,8-bis(2-isooctyl-thienyl)-benzo(1,2-b; 4,5-b′)-dithiophene-2,5-diisooctyloxy-3,6- Preparation of bis(furan-2-methylene)pyrazine (P1).
[0055] Specific steps are as follows:
[0056] ①Synthesis of Compound 1
[0057] 1,4-Diacetylpiperazine-2,5-dione (1.19g, 6mmol) and 5-bromo-2 furfural (2.63g, 15mmol) were placed in a 50ml single-necked round bottom flask, and then 28ml DMF, under the protection of nitrogen, heat and stir, raise the temperature to 120°C, add 3.32ml of triethylamine through a syringe, after the completion, the reaction system turns from colorless to red immediately, continue to react for 12 hours, after the completion of the reaction, cool to room temperature, produce The yellow precipitate was collected by filtration, washed with acetone, and the product was collected to obtain the target product (2.16 g, yield: 84%), which was directly carried out to the next reaction without further purification.
[0058] The nuclear magnetic resonance spectrum ...
Embodiment 2
[0071] Example 2 4,8-bis(2-isooctyl-thienyl)-benzo(1,2-b; 4,5-b')-difuran-2,5-dioctyloxy-3, Preparation of 6-bis(furan-2-methylene)pyrazine (P2)
[0072] The synthetic method of compound 2 is the same as the synthetic method of compound 2 in embodiment 1, and brominated alkane adopts brominated n-octane, and concrete steps are as follows:
[0073] ①Synthesis of Compound 1
[0074] 1,4-Diacetylpiperazine-2,5-dione (1.19g, 6mmol) and 5-bromo-2 furfural (2.63g, 15mmol) were placed in a 50ml single-necked round bottom flask, and then 28ml DMF, under the protection of nitrogen, heat and stir, raise the temperature to 120°C, add 3.32ml of triethylamine through a syringe, after the completion, the reaction system turns from colorless to red immediately, continue to react for 12 hours, after the completion of the reaction, cool to room temperature, produce The yellow precipitate was collected by filtration, washed with acetone, and the product was collected to obtain the target prod...
Embodiment 3
[0088] Example 3 4,8-bis(2-isooctyl-thienyl)-benzo(1,2-b; 4,5-b')-difuran-2,5-bis(2-octyldec Preparation of dialkoxy)-3,6-bis(furan-2-methylene)pyrazine (P3)
[0089] The synthetic method of compound 2 is the same as the synthetic method of compound 2 in embodiment 1, and brominated alkane adopts brominated 2-octyl-dodecane, and concrete steps are as follows:
[0090] ①Synthesis of Compound 1
[0091] 1,4-Diacetylpiperazine-2,5-dione (1.19g, 6mmol) and 5-bromo-2 furfural (2.63g, 15mmol) were placed in a 50ml single-necked round bottom flask, and then 28ml DMF, under the protection of nitrogen, heat and stir, raise the temperature to 120°C, add 3.32ml of triethylamine through a syringe, after the completion, the reaction system turns from colorless to red immediately, continue to react for 12 hours, after the completion of the reaction, cool to room temperature, produce The yellow precipitate was collected by filtration, washed with acetone, and the product was collected to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com