Method for Rapid Culture and Differentiation of Adipose Stem Cells
An adipose stem cell and cell technology, applied in the field of stem cells, can solve the problems of endotoxin, cell activity needs to be strengthened, and fat stem cells cannot be obtained.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
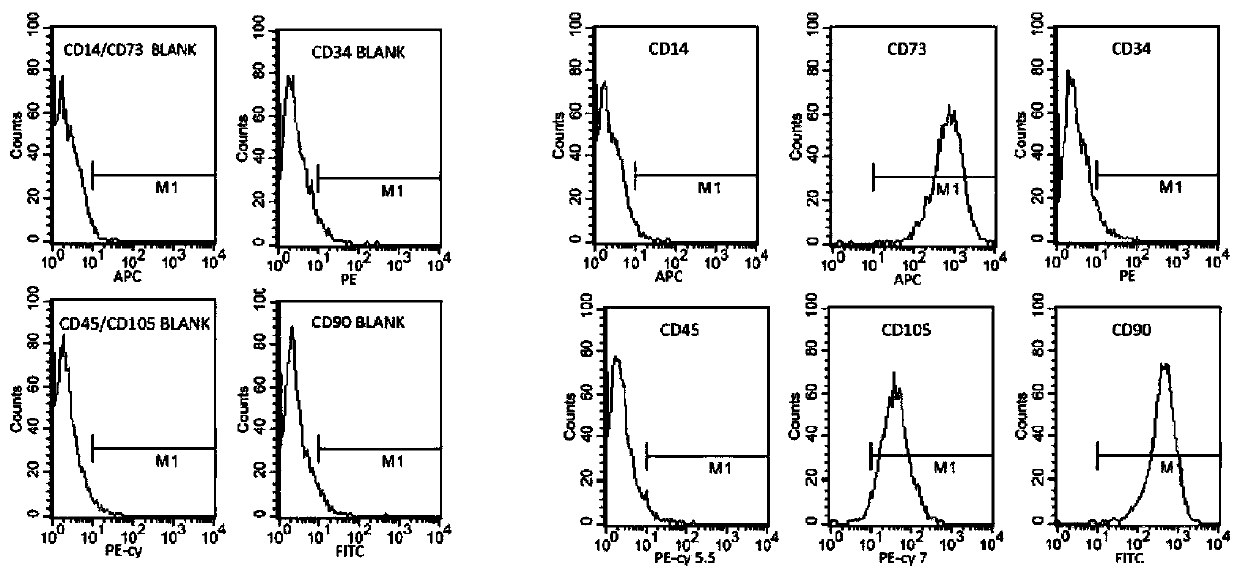
[0020] Example 1 Separation of Adipose Stem Cells
[0021] The subcutaneous adipose tissue of healthy volunteers was taken, washed with PBS buffer 2 to 3 times, and the adipose tissue was cut into pieces of 0.5 mm under sterile conditions. 2The tissue block was digested by adding 0.075% collagenase type Ⅰ and 0.1% trypsin at 2 times the volume at 37°C for 1 hour with shaking at 20 r / min to carry out digestion and separation. Filter through a 70 μm cell sieve, centrifuge at 1500 r / min for 10 min at 4°C, and discard the supernatant. The erythrocyte lysate was treated for 5 minutes, PBS was added, centrifuged at 1500 r / min for 10 minutes at 4°C, and the supernatant was discarded. Inoculate in a culture flask at a density of 4000 cells / cm2, and the culture medium is DMEM containing 10% fetal bovine serum and 0.05% cell activity stimulating peptide (SEQ ID NO: 1). After 48 hours, the medium was changed for the first time to remove non-adherent cells and residual red blood cells. ...
Embodiment 2
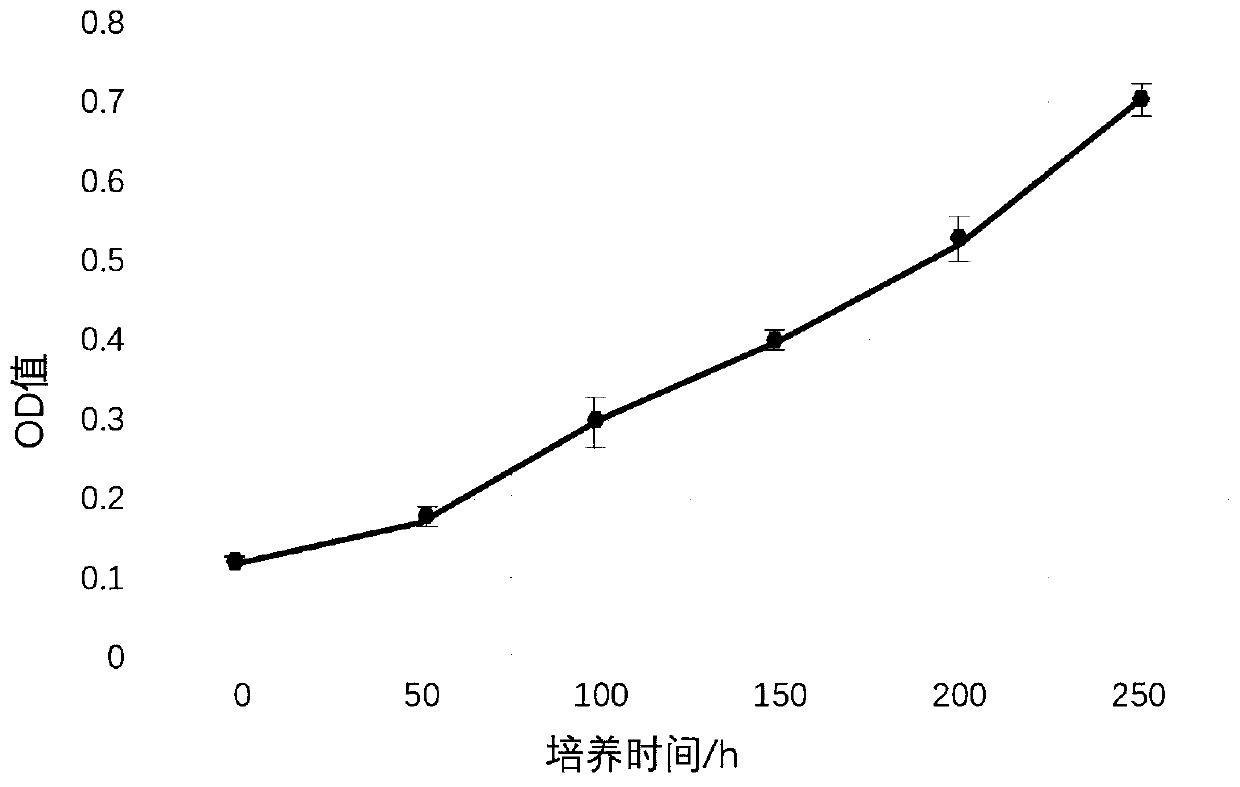
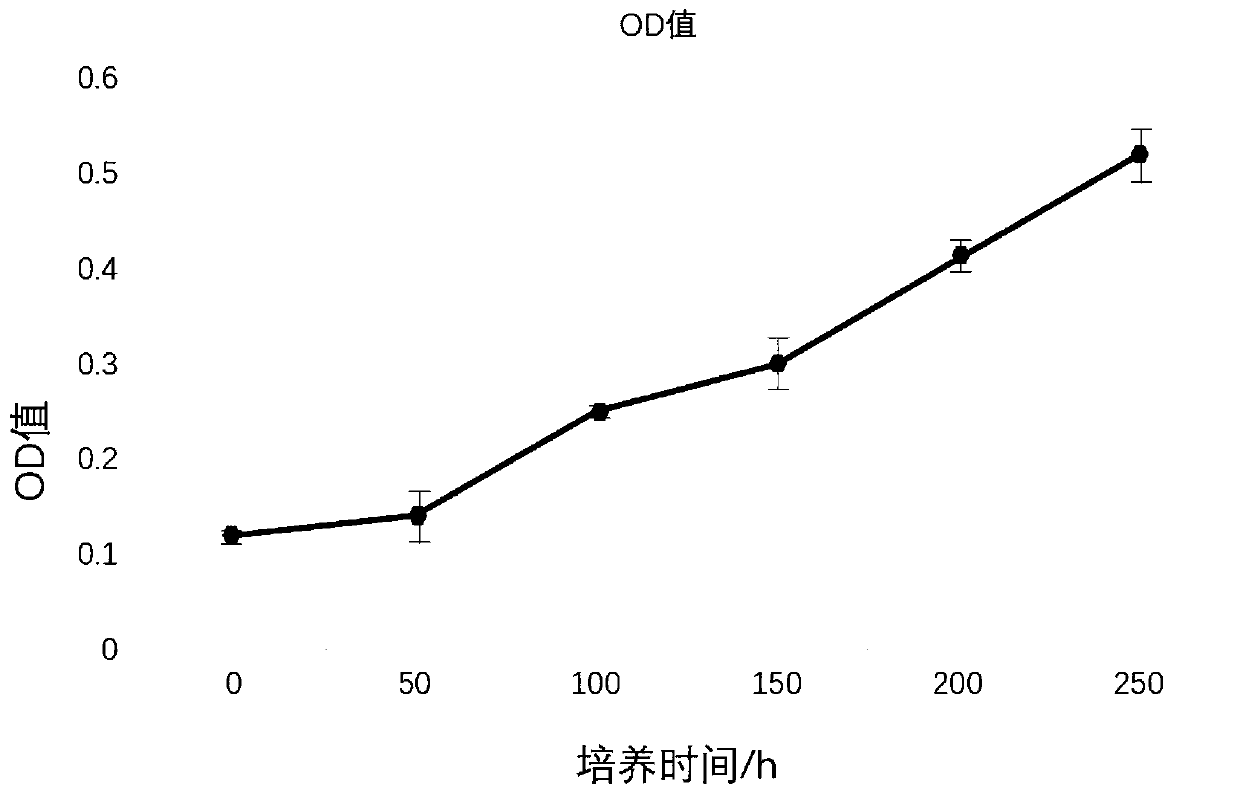
[0022] The proliferation speed experiment of embodiment 2 cells
[0023] Using the MTT method, the P3, P4, and P5 generation cells in the logarithmic growth phase were inoculated in a 96-well plate at 4000 cells / well, divided into 4 groups, and each group had three replicate wells, and cultured in a 37°C, 5% CO2 incubator. Add MTT solution (5mg / mL) every 24h, continue to incubate for 4h, terminate the culture, and discard the culture supernatant. Add 150 μL DMSO to each well and shake for 10 minutes to fully melt the crystals. Colorimetry: select 490nm wavelength, measure the light absorption value of each hole on the enzyme-linked immunosorbent monitor, record the result, take the time as the abscissa, and the light absorption value as the ordinate to draw the cell growth curve, and use the culture medium without polypeptide as a contrast, from figure 2 It can be seen that the adipose stem cell culture method prepared by the present invention has a relatively fast cell cul...
Embodiment 3
[0024] Example 3 Identification of Inducible Differentiation Ability of Adipose Stem Cells
[0025] DMEM containing 10 nM dexamethasone (Sigma, St Louis, MO, USA) and 15 mg / L hydroxyapatite was used to induce osteogenic differentiation of human adipose-derived stem cells at passage 16 for 24 h. The next day the cells were replaced with osteogenic culture medium: cell culture medium DMEM+10% fetal bovine serum, 10mM 3-phosphate glyceraldehyde, 60mM ascorbic acid, 1OnM dexamethasone, 0.05% cell activity stimulating peptide SEQ ID NO: 1 and 15mg / L hydroxyapatite. The osteogenic culture medium was replaced every two days, and cultured for 8-14 days. The inducibility without peptide was used as positive control, and DMEM without inducer and fetal bovine serum were used as negative control. Analysis of qRT-PCR results Through the detection of induced ADSCs gene expression, the influence of ADSCs osteogenesis-related gene expression was analyzed. The results are shown in Table 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com