Probe and application thereof
A probe and light reaction technology, applied in the biological field, can solve the problem of low efficiency of signal transduction complexes, and achieve the effect of effective extraction and enrichment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
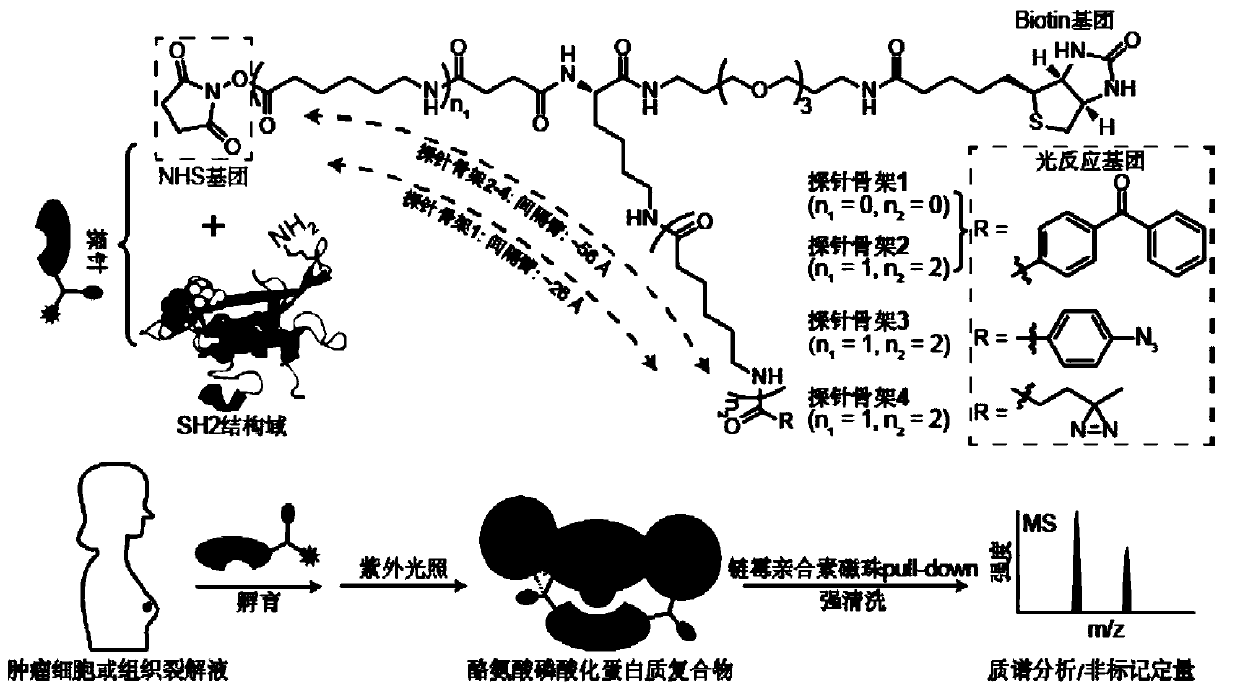
[0093] Embodiment 1 Probe backbone 2 labels SH2 bait protein
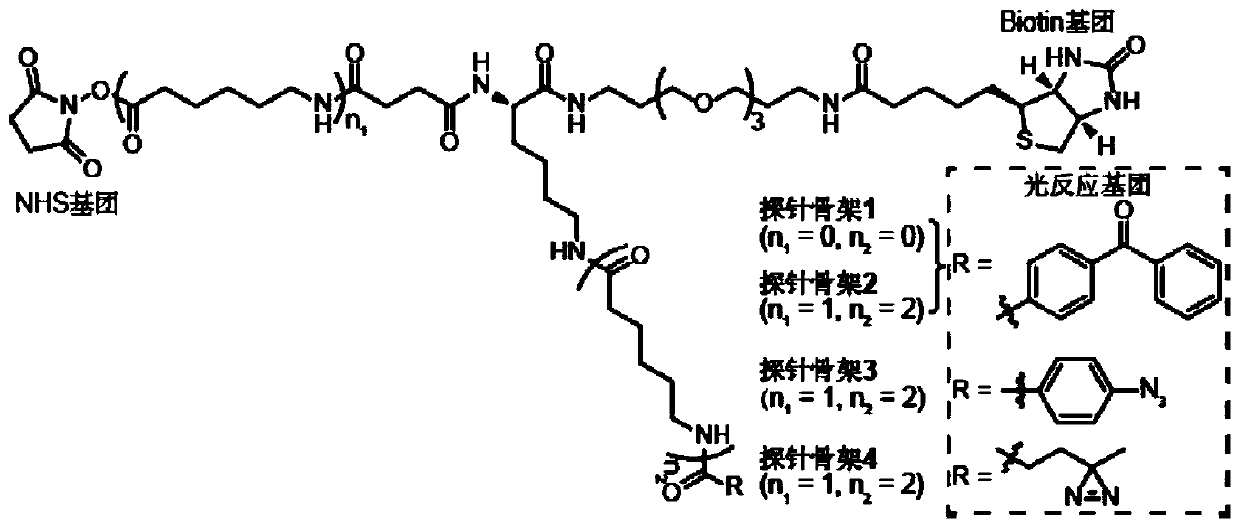
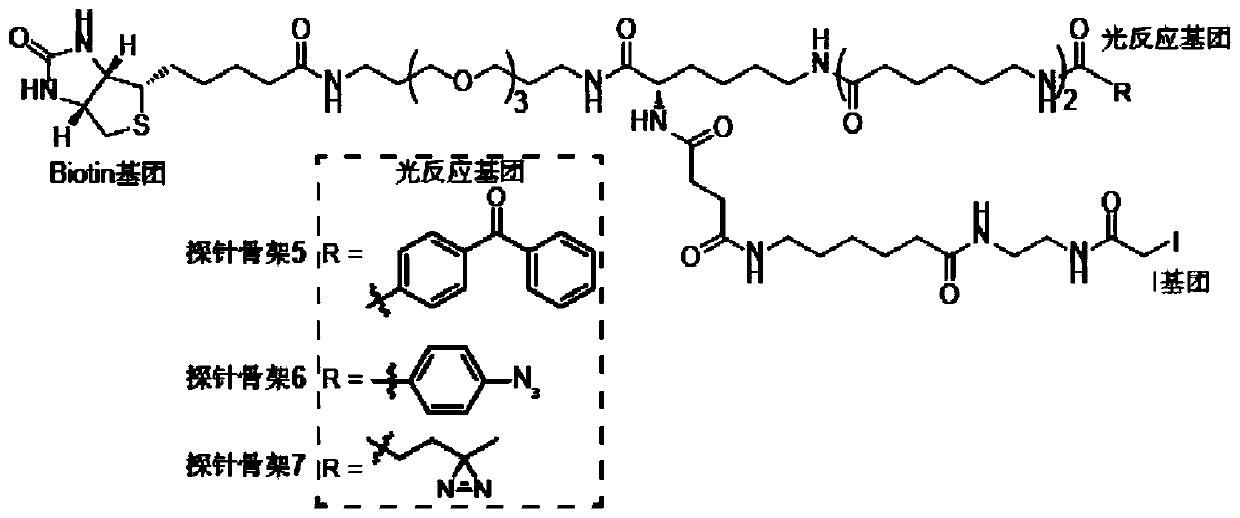
[0094] The present invention designs 7 kinds of probe skeletons, which are used to prepare such as Figure 1A The functional probe shown has high-efficiency enrichment and identification of tyrosine phosphorylated protein complexes. Among them, the structure of the probe skeleton is as follows Figure 1B with Figure 1C shown.
[0095] To 50 μL SH2 domain (1 mg / mL, dissolved in HEPES buffer), add 0.5 μL probe backbone containing photoreactive groups and biotin groups (probe backbone dissolved in DMSO, SH2 domain and The molar ratio of the probe skeleton is 1:10), reacted at room temperature for 1 min, then added 5 μL of 1 mol / L glycine solution to terminate the activity of the NHS group, and obtained the following Figure 1A Probes labeled with the SH2 domain of the bait protein are shown.
Embodiment 2
[0096] Example 2 Mass spectrometry sample pretreatment
[0097] (1) According to the method in Example 1, using probe skeletons 1-7 to prepare probes with different spacer arm lengths or photoreactive groups, add 1.4 mg of cell lysate, incubate slowly at 4 °C for 2 h, and then 365 nm ultraviolet light Light for 30min;
[0098] (2) Add 30 μL of streptavidin microspheres, incubate slowly at 4°C for 2 h, wash with 1 mL of mRIPA buffer three times, and wash once with 1 mL of 50 mM ammonium bicarbonate (ABC).
Embodiment 3
[0099] Embodiment 3 prepares mass spectrometry detection sample
[0100] (1) Incubation and digestion:
[0101] Add Tris(2-carboxyethyl)phosphine hydrochloride (Tris(2-carboxyethyl)phosphine hydrochloride, TCEP, dissolved in 50mM ABC) with a final concentration of 10mM to the sample pretreated in Example 2, and incubate at room temperature for 15min; Iodoacetamide (iodoacetamide, IAA, dissolved in 50 mM ABC) with a final concentration of 30 mM was incubated at room temperature for 15 min in the dark; TCEP with a final concentration of 5 mM was added and incubated at room temperature for 15 min; the sample was washed once with 1 mL of 50 mM ABC, and the Add 50 μL of trypsin solution (trypsin, 2 μg, dissolved in 50 mM ABC), and enzymatically digest at 37 ° C;
[0102] (2) Enzymatic peptide desalination:
[0103] 1) Collect the enzymolyzed polypeptide: centrifuge to collect the above-mentioned overnight enzymatically hydrolyzed sample, add 50 μL of 1% (v / v) formic acid, wash th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com