Duck tembusu virus genetic engineering subunit vaccine and preparation method and application thereof
A duck Tembusu virus and amino acid technology, applied in genetic engineering, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of strong virulence, further research, and poor protein biological activity. Achieve high expression level, reduce production cost, and strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
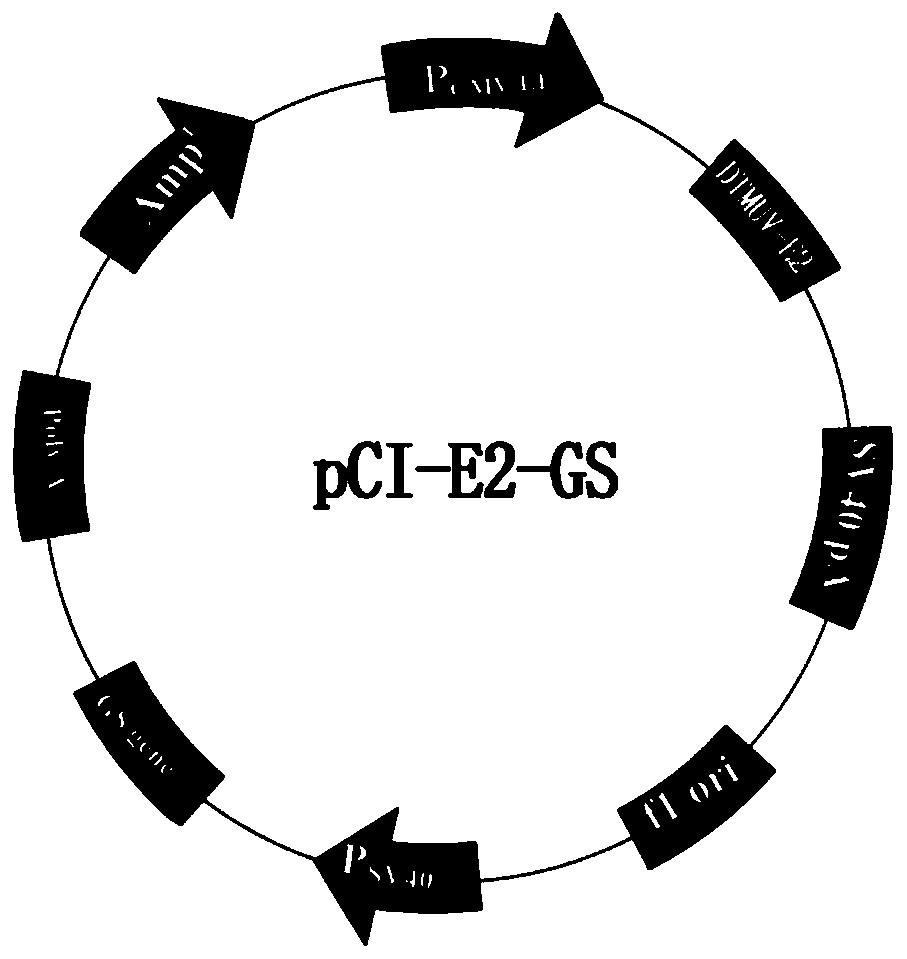
[0109] Example 1 Construction of recombinant eukaryotic expression vector pCI-E2-GS
[0110] 1. The codon-optimized DTMUV E2 gene was obtained from Nanjing GenScript Biotechnology Co., Ltd., and cloned into the pUC-57 vector to construct the pUC-E2 plasmid vector. The optimized E2 gene sequence is shown in SEQ ID NO:1.
[0111] 2. E2 gene amplification uses pUC-E2 as a template, and E2-F and E2-R as primers for PCR amplification (the gene sequences of E2-F and E2-R are shown in SEQ ID NO: 3 and 4), and the amplified See Table 1 for the augmentation system. The reaction conditions were: pre-denaturation at 94°C for 5 minutes; denaturation at 95°C for 45 seconds, renaturation at 60°C for 45 seconds, extension at 72°C for 2 minutes, 30 cycles; extension at 72°C for 10 minutes, and storage at 4°C.
[0112] Table 1 E2 gene amplification system
[0113]
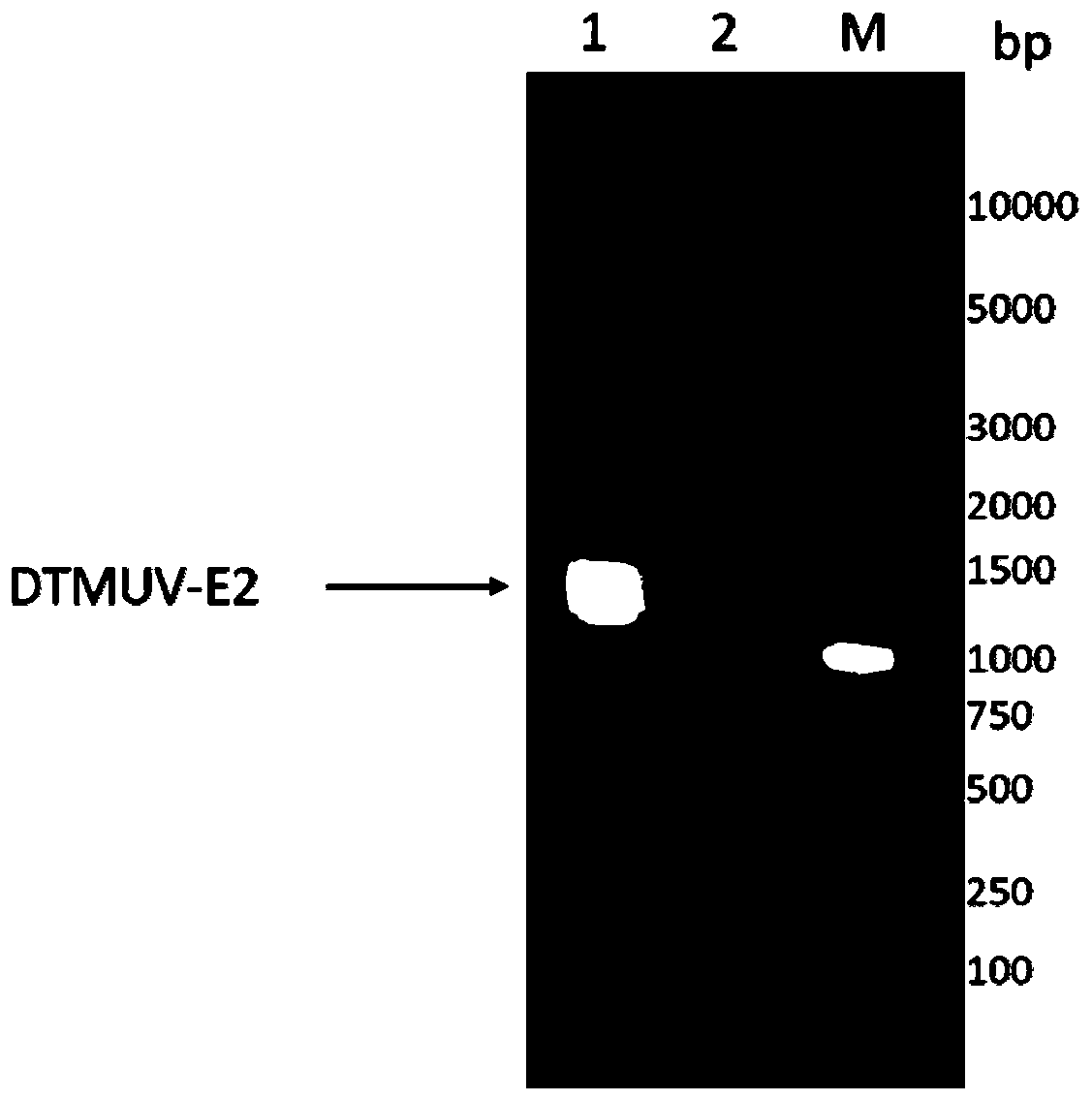
[0114] Perform gel electrophoresis on the PCR product to identify the size of the target gene, such as figure 1 As shown, ...
Embodiment 2
[0126] Example 2 Construction and screening of recombinant CHO cells expressing E2 protein
[0127] 1. Cell Transfection
[0128] 1.1 Prepare cells Take CHO cells in the logarithmic growth phase, sample and count, and use 1×10 6 The cell density of cells / ml continues to be subcultured, maintain the seeds, centrifuge the remaining cells, centrifuge at 1000rpm for 4 minutes, discard the supernatant, resuspend with about 20ml of fresh CHO-WM medium, centrifuge again, centrifuge at 1000rpm for 4 minutes, discard the supernatant After resuspending with a small amount of medium for counting, the final cell density was adjusted to 1.43×10 7 cells / ml.
[0129] 1.2 Plasmid and cell mixing Take 5ug of the pCI-E2-GS plasmid vector in Example 1, add it to the EP tube, add 0.7ml cells, mix well, and let stand for 15 minutes.
[0130] 1.3 Electroporation 280V 20ms electric shock for 2 pulses. Immediately after the electric shock is completed, the cells are transferred to a shaker flask a...
Embodiment 3
[0140] Example 3 SDS-PAGE detection
[0141] The cell culture supernatant harvested in Example 2 was subjected to SDS-PAGE detection, and empty CHO cells were used as a negative control. The specific operation is as follows: take 40 μl of the harvested cell culture, add 10 μl of 5× sample buffer, bathe in boiling water for 5 minutes, centrifuge at 12000 r / min for 1 minute, and take the supernatant for SDS-PAGE gel (12% concentration gel) Electrophoresis. After electrophoresis, the gel was stained and decolorized to observe the target band.
[0142] The detection result of culture in embodiment 2 is as Figure 4 As shown, the target band appears around the molecular weight of about 53kDa, and the negative control has no band at the corresponding position.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com