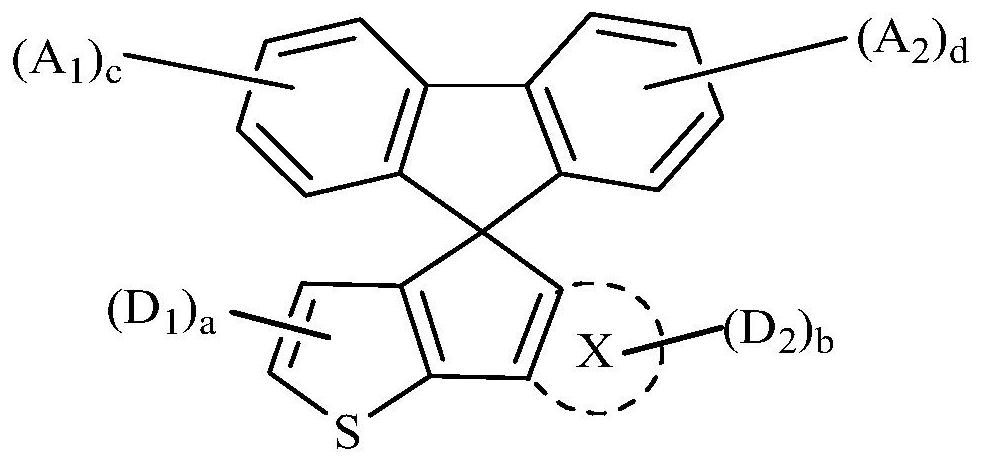
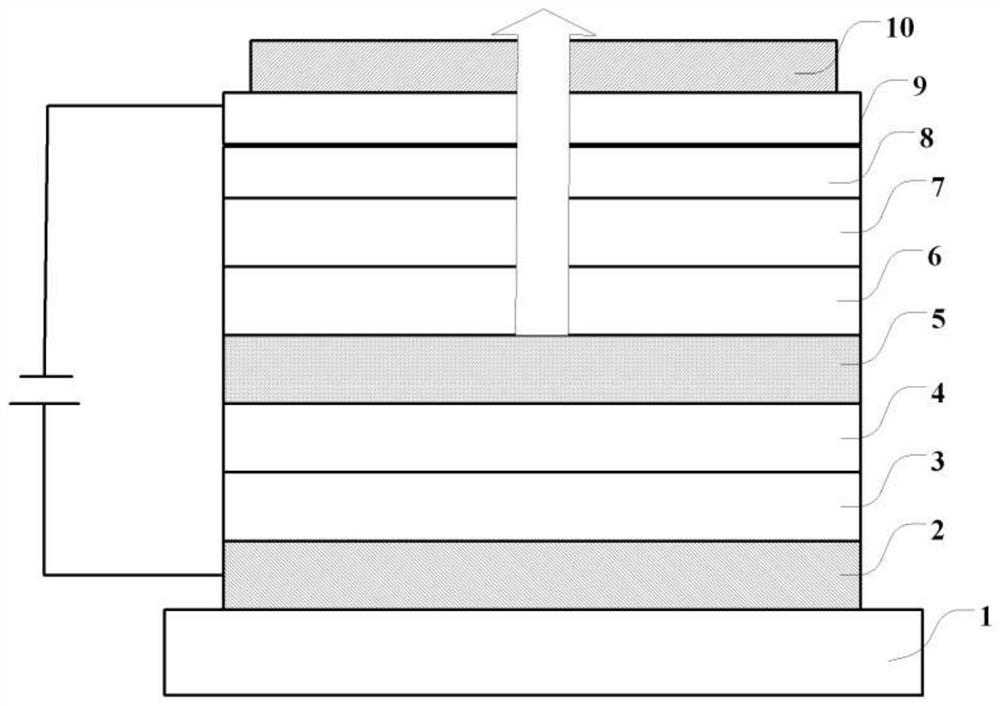
compounds, oled devices and electronic devices
A technology of electronic devices and compounds, applied in the field of OLED, can solve the problems of the efficiency roll-off of phosphorescent materials and the poor stability of phosphorescent devices, and achieve good charge transport performance and electron donating performance, good polarizability, and unique electrical properties. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Synthesis of compound H01
[0104]
[0105] S1 (2.7mmol), S2 (5.7mmol), (dibenzylideneacetone) dipalladium (0) (0.35mmol), sodium tert-butoxide (10.0mmol), tri-tert-butylphosphine tetrafluoroborate (0.7mmol ) into a 500mL three-necked flask, and while stirring, quickly repeated degassing and nitrogen replacement 3 times, and added 150mL toluene through a syringe. The mixture was heated to reflux for 3 hours under nitrogen flow. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain S3 (2.05 mmol, 76%).
[0106]MALDI-TOF MS: Calculated m / z: C 35 h 20 N 2 OS: 516.1; Measured: 516.2.
[0107]
[0108] Under a nitrogen atmosphere, S4 (6.9 mmol) was dissolved in 60 ml of anhydrous THF, and the reaction system was stirred a...
Embodiment 2
[0115] Synthesis of Compound H29
[0116]
[0117] S7 (3.5mmol), S8 (7.4mmol), (dibenzylideneacetone) dipalladium (0) (0.5mmol), sodium tert-butoxide (12.0mmol), tri-tert-butylphosphine tetrafluoroborate (1.0mmol ) into a 500mL three-necked flask, and while stirring, quickly repeated degassing and nitrogen replacement 3 times, and added 180mL toluene through a syringe. The mixture was heated to reflux for 3 hours under nitrogen flow. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain S9 (2.56 mmol, 73%).
[0118] MALDI-TOF MS:C 47 h 24 N 2 OS 3 m / z calculated: 728.1; measured: 728.2.
[0119]
[0120] Under a nitrogen atmosphere, S4 (5.5 mmol) was dissolved in 45 ml of anhydrous THF, and the reaction system was stirred...
Embodiment 3
[0127] Synthesis of compound H49
[0128]
[0129] S12 (4.2mmol), S2 (8.5mmol), (dibenzylideneacetone) dipalladium (0) (0.6mmol), sodium tert-butoxide (14.4mmol), tri-tert-butylphosphine tetrafluoroborate (1.2mmol ) into a 500mL three-necked flask, and while stirring, quickly repeated degassing and nitrogen replacement 3 times, and added 160mL toluene through a syringe. The mixture was heated to reflux for 3 hours under nitrogen flow. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain S13 (2.9 mmol, 69%).
[0130] MALDI-TOF MS: Calculated m / z: C 33 h 18 N 2 OS 2 : 522.1; measured value: 522.2.
[0131]
[0132] Under a nitrogen atmosphere, S14 (2.8 mmol) was dissolved in 25 ml of anhydrous THF, and the reaction system w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com