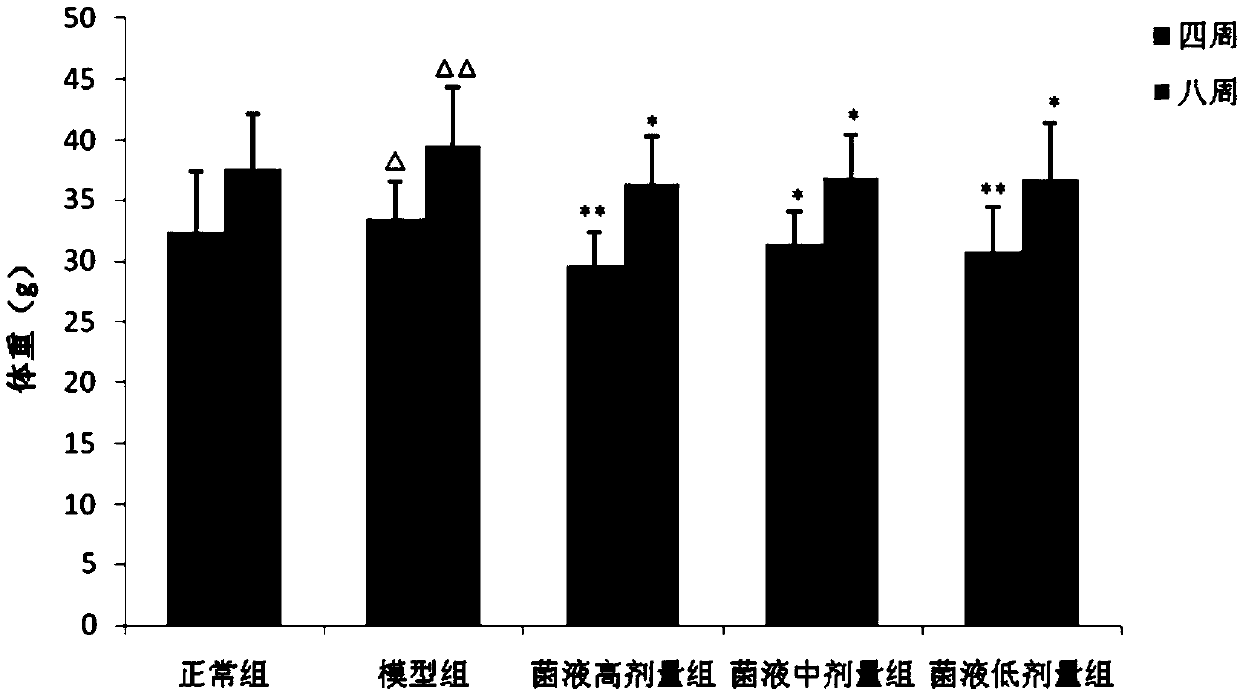
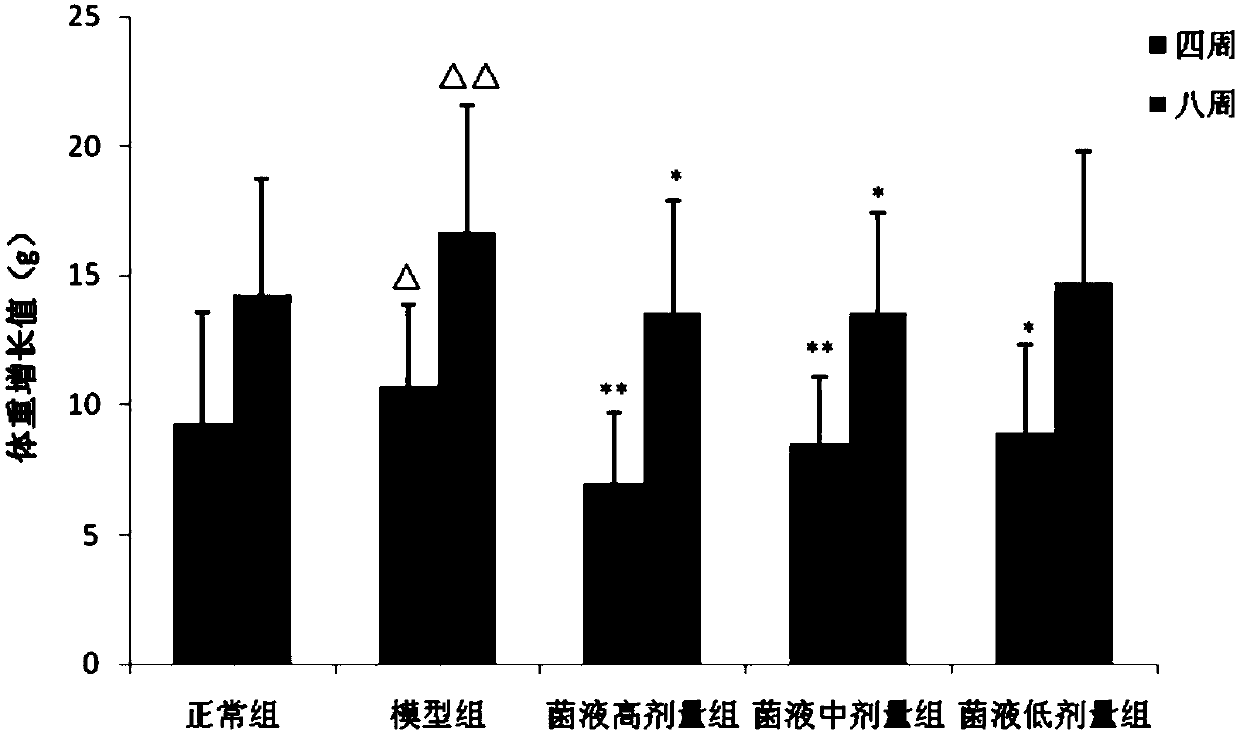
Drug having weight-loss function
A drug and application technology, applied in the field of GLP-1 mutants, can solve problems such as irritation and allergic reactions, physical and psychological pain of patients, and inconvenient use and carrying, so as to reduce degradation, continuously control body weight, and avoid painful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 The transformation of the original sequence of GLP-1 in the present invention
[0032] Starting from the original sequence of GLP-1, the mutant polypeptide sequence is modified and designed, and a polypeptide product with a purity of up to 85% is obtained through chemical synthesis (Zhongpeptide Biochemical). The peptide sequence is as follows:
[0033] GLP-1 Fragment (7-37aa): HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG
[0034] GM (SEQ ID NO: 1): HHEGTFTSDVSSYLEGQAAKKFIAWLVRGGKKKKKYGRKK RRQRRREF
[0035] GM is based on the original sequence of GLP-1, mutating the 8th alanine of GLP-1 to histidine, the 27th glutamic acid to lysine, and the 34th lysine to arginine acid, arginine at position 36 is mutated to glycine, glycine at position 37 is mutated to lysine, and a sequence is added at the end.
[0036] The differences between the original sequence of GLP-1 and the mutant GM are shown in Table 1.
[0037] Table 1 Sequence comparison of GLP-1 and mutant GM
[0038...
Embodiment 2
[0041] Embodiment 2 Mutant GM transforms Escherichia coli Nissle1917
[0042] 1. Add a signal peptide sequence before the sequence of GLP-1 mutant GM, add HIV membrane-penetrating peptide sequence and 6 His-tag sequences at the 3'-end, and construct GLP that can be secreted and expressed and has the ability to pass through the cell membrane -1 mutant, its nucleotide sequence is shown in SEQ ID NO:2.
[0043] SEQ ID NO: 2:
[0044] atgaaaaagaacatcgcattcctcctggcatctatgtttgttttctctatcgctaccaacgcttacgctggatcccaccacgagggcaccttca cctccgacgtgtcctcctacctggagggccaggccgccaagaagttcatcgcctggctggtgcgcggcggcaagaagaagaagaagt acggccgcaagaagcgccgccagcgccgccgcctcgaggacgacgacgacaagcaccatcaccatcaccattaa
[0045] 2. Cloning the nucleotide sequence shown in SEQ ID NO: 3 into the BamHI and SalI sites of the Escherichia coli Nissle1917 expression vector pGEX to obtain the pGEX-GLP-1GM vector, and transform it into E. coli E.coli BL21(DE3 ), the target fragment size is about 12KD. After purificatio...
Embodiment 3
[0047] The preparation of embodiment 3 mutant GM transformation Escherichia coli Nissle1917 preparation
[0048] 1. Preparation of mutant GM transformed Escherichia coli Nissle1917 oral solution
[0049] After adding erythromycin-resistant LB liquid medium with a final concentration of 200 μg / ml for steam sterilization, Escherichia coli Nissle1917 (prepared in Example 2), which was stably transformed with a gene identified by PCR verification and induction expression experiment, was inoculated at 37° C. Cultivate on a shaker at 250r / min until the OD600 value reaches 0.8-1.0, then centrifuge for 5 minutes to harvest the bacteria, wash them twice with normal saline, collect the bacterial precipitate by centrifugation, and add L-arabinose solution with a final concentration of 2% (w / v) Or 20% fructooligosaccharide solution to resuspend the bacteria, prepared to be not less than 10 per milliliter 8 The fresh bacterial solution of viable bacteria was stored in a refrigerator at 4°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com