Synthesis method of ethyl N-cyanoethanimideate
A technology of ethyl cyanoethylimidate and ethyl ethylimidate hydrochloride, applied in the direction of organic chemistry, etc., can solve production costs, high energy consumption, difficult to handle by-products, low yield, low purity, etc. problem, to achieve the effect of increasing the content, avoiding inseparability, and simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
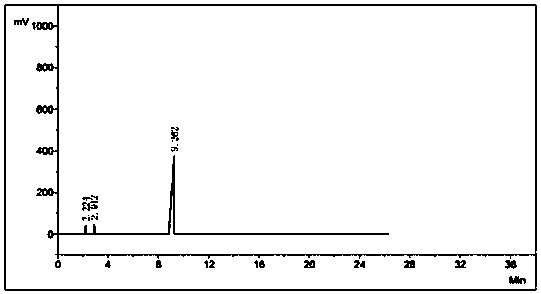
[0038] Add 505.1 g of ethyl imidate hydrochloride in a stirred reactor, add 544.0 g of cyanamide aqueous solution with a mass concentration of 30% under stirring, stir for 3 minutes to make ethyl imidate hydrochloride After the acid salt is fully dissolved, start to add dropwise a mass percent concentration of 30% sodium hydroxide aqueous solution, adjust the pH value of the solution in the reactor to about 6.5, and after reacting for 60 minutes at normal temperature, get the organic phase for gas chromatography analysis. The ester content is: 97.2%.
[0039] After the reaction, the system is poured into a separatory funnel at room temperature to allow it to be layered. The lower layer is an aqueous phase, and the upper layer is an organic phase. The organic phase can be distilled under reduced pressure to obtain high-purity ethyl N-cyanoethylimidate. Ester product, the content of cyanoethyl ester in the final product is: 99.8%, total yield: 83%; After the water phase remainin...
Embodiment 2
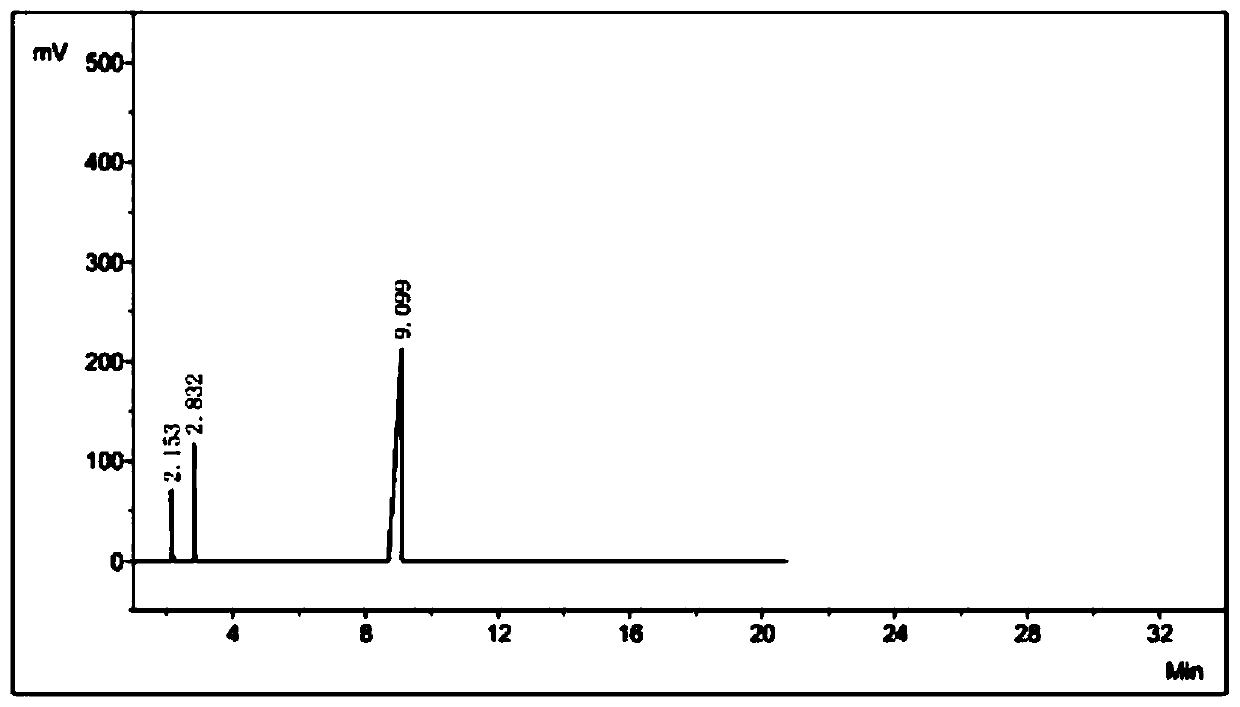
[0041] Add 505.1 g of ethyl acetimidate hydrochloride in a stirred reactor, add 524.1 g of cyanamide aqueous solution with a mass concentration of 25% under stirring, stir for 3 minutes to make ethyl acetimidate hydrochloride After the acid salt is fully dissolved, start to add dropwise a mass percent concentration of 10% sodium hydroxide aqueous solution, adjust the pH value of the solution in the reactor to about 5, and react at normal temperature for 10 minutes, get the organic phase for gas chromatography analysis, and the cyanoethyl The ester content is: 86.9%.
[0042] After the reaction, the system is poured into a separatory funnel at room temperature to allow it to be layered. The lower layer is an aqueous phase, and the upper layer is an organic phase. The organic phase can be distilled under reduced pressure to obtain high-purity ethyl N-cyanoethylimidate. Ester product, the content of cyanoethyl ester in the final product is: 99.6%, total yield: 72%; After the wate...
Embodiment 3
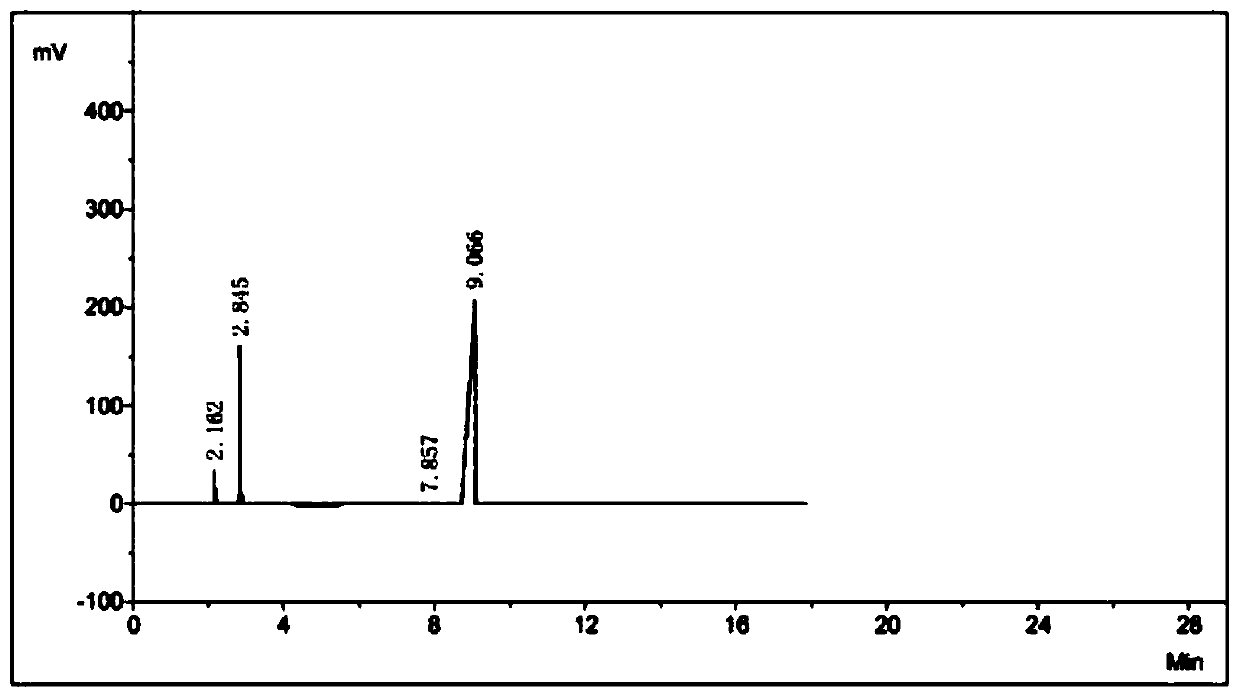
[0044] Add 505.1 g of ethyl imidate hydrochloride in a stirred reactor, add 468.2 g of cyanamide aqueous solution with a mass concentration of 35% under stirring, stir for 3 minutes to make ethyl imidate hydrochloride After the acid salt is fully dissolved, start to add dropwise a mass percent concentration of 20% sodium hydroxide aqueous solution, adjust the pH value of the solution in the reaction kettle to about 6, and after reacting for 30 minutes at normal temperature, get the organic phase for gas chromatography analysis. The ester content is: 93.6%.
[0045] After the reaction, the system is poured into a separatory funnel at room temperature to allow it to be layered. The lower layer is an aqueous phase, and the upper layer is an organic phase. The organic phase can be distilled under reduced pressure to obtain high-purity ethyl N-cyanoethylimidate. Ester product, the content of cyanoethyl ester in the final product is: 99.4%, total yield: 78%; After the water phase re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com