Preparation method of gliquidone intermediate
A technology for gliquidone and intermediates, applied in the field of pharmaceutical chemical synthesis, can solve problems such as low product purity, long preparation time, waste of resources, etc., achieve the effects of improving product purity, reducing purification steps, and solving environmental problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
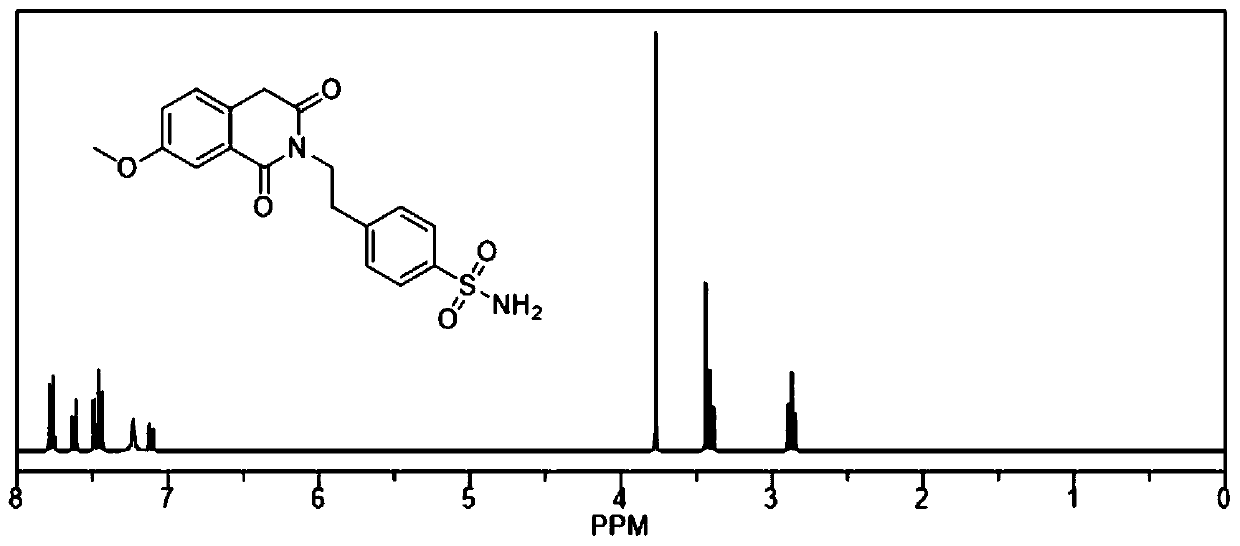
[0021] The present invention relates to the preparation method of gliquidone intermediate, its structural formula is shown in formula (1),
[0022]
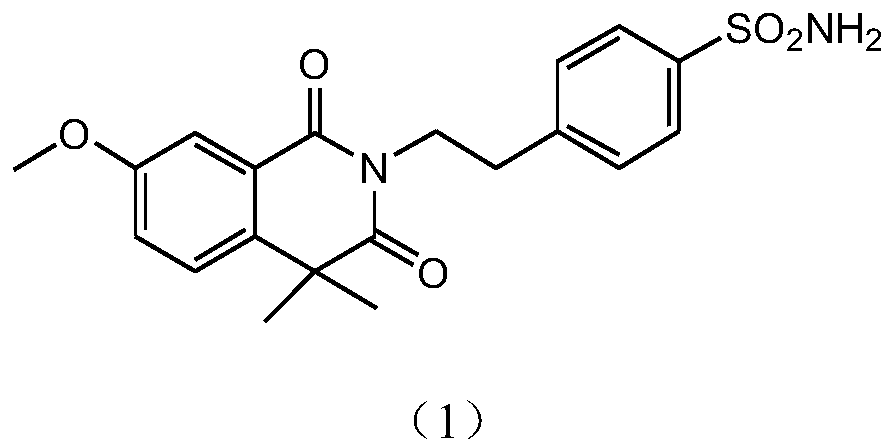
[0023] This scheme realizes the process of preparing the intermediate of gliquidone through a one-step method, specifically, 7-methoxy-2,4,4-trimethyl-1,3(2H,4H)-isoquinolinedione is used as Raw material, react with sodium hydroxide and 4-(2-aminoethyl)-benzenesulfonamide in xylene solution to prepare 4-(2-(3,4-dihydro-7-methoxy-4, 4'-Dimethyl-1,3-dioxo-2(1H)-isoquinolinyl)ethyl)benzenesulfonamide, the structural formulas of the compound of formula (2) and the compound of formula (3) are as follows,
[0024] Wherein, the structural formula of 7-methoxy-2,4,4-trimethyl-1,3(2H,4H)-isoquinolinedione is shown in formula (2),
[0025]
[0026] 4-(2-aminoethyl)-benzenesulfonamide structural formula is shown in formula (3),
[0027]
[0028] The specific preparation method is: sequentially add 7-methoxy-2,4,4-trimethyl-1,3(2H...
Embodiment 1
[0033] Add 300ml of xylene, 20g of 7-methoxy-2,4,4-trimethyl-1,3(2H,4H)-isoquinolinedione raw material, 0.17g of sodium hydroxide and 15.45 g4-(2-aminoethyl)-benzenesulfonamide raw material, start stirring, control the temperature at 100°C, add 1g of water dropwise, and the dropwise addition is completed in 15 minutes; heat to 140°C and stir for 4h, change to distillation, and distill out the distillate After adding toluene, add 300ml of n-propanol to the reaction system and heat to 80°C to dissolve. Cool down to 0-5°C, stir and crystallize for 3-5h, filter, filter, wash the filter cake, and dry to obtain 4-(2-(3,4-dihydro-7-methoxy-4,4'-di Methyl-1,3-dioxo-2(1H)-isoquinolyl)ethyl)benzenesulfonamide finished product. The melting point of the light yellow reaction product was tested, and the m.p. was 200°C. Compared with 4-(2-(3,4-dihydro-7-methoxy-4,4'-dimethyl- The melting point of 1,3-dioxo-2 (1H)-isoquinolyl) ethyl) benzenesulfonamide is consistent; In addition, the light...
Embodiment 2
[0035] Add 300ml of xylene, 20g of 7-methoxy-2,4,4-trimethyl-1,3(2H,4H)-isoquinolinedione raw material, 0.17g of sodium hydroxide and 16.31 g4-(2-Aminoethyl)-benzenesulfonamide raw material, start stirring, control the temperature at 100°C, add water dropwise, and the dropwise addition is completed in 10-20min; heat to 140°C and stir for 4h, change to distillation, and distill out distillate After adding toluene, add 300ml of n-propanol to the reaction system and heat until dissolved. Cool down to 0-5°C, stir and crystallize for 3-5h, filter, filter, wash the filter cake, and dry to obtain 4-(2-(3,4-dihydro-7-methoxy-4,4'-di Methyl-1,3-dioxo-2(1H)-isoquinolyl)ethyl)benzenesulfonamide finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com