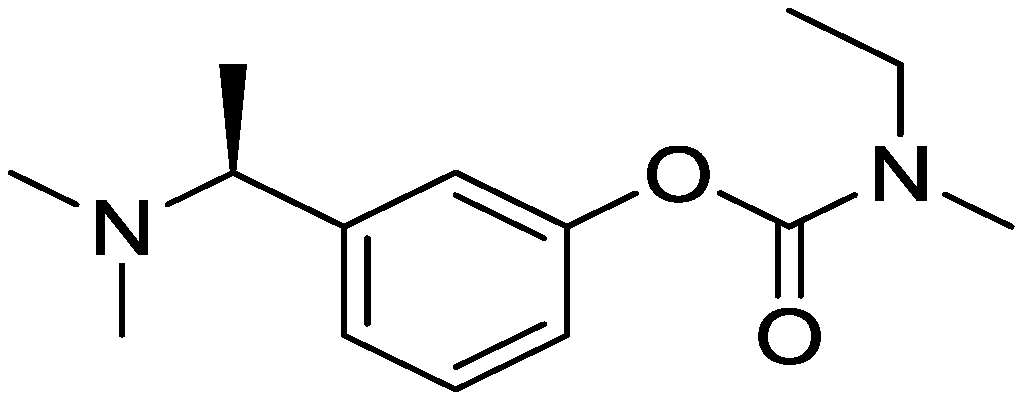
Preparation method of rivastigmine citrate
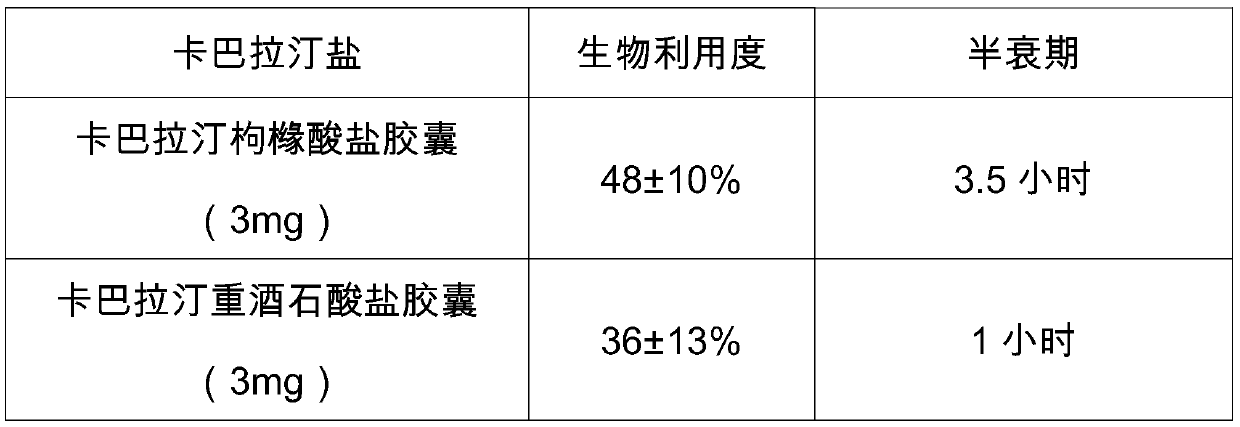
A technology of citrate and anhydrous sodium carbonate, which is applied in the direction of carboxylate preparation, carboxylate preparation, sulfonate preparation, etc., can solve the problem of difficult to obtain high-purity rivastigmine, complex synthesis steps and cost Advanced issues, achieve the effect of easy realization of reaction conditions and controllability, improvement of bioavailability, and improvement of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of 3-acetylphenyl N-ethyl-N-methylcarbamate
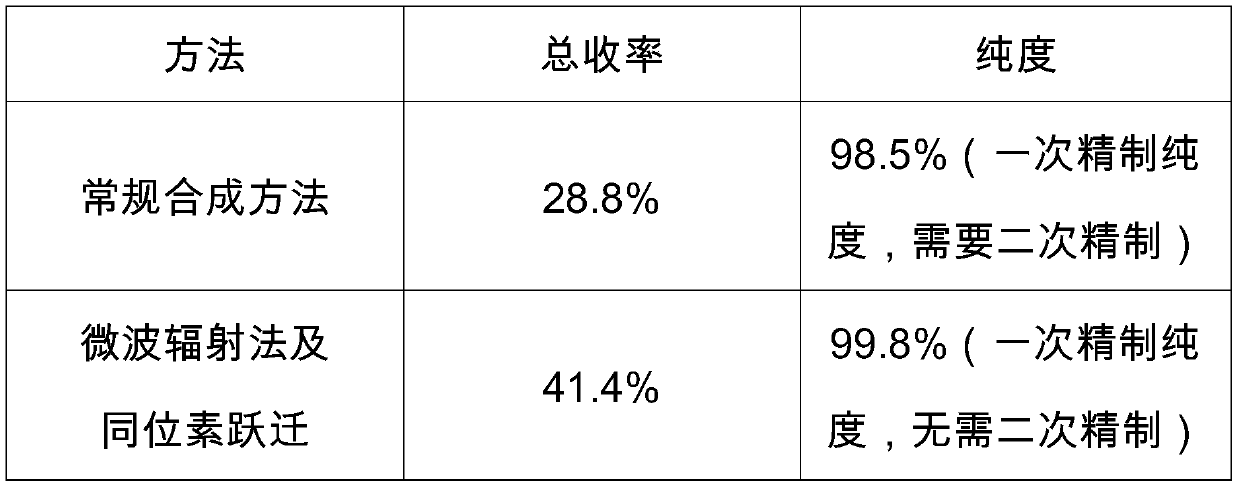
[0042]Under the protection of argon, 3.58 mol of m-hydroxyacetophenone was taken in a microwave reactor, dissolved in THF solution, 6.88 mol of anhydrous sodium carbonate was added under stirring, and 4.26 mol of methylethylcarbamoyl chloride was slowly added dropwise after stirring and dissolving. Control the adding time at about 30min, heat to reflux, turn on the microwave frequency and set it to 3600MHz, react for 8-10h, detect with TLC, track until the raw material point completely disappears, filter out the solid, add 15L of water and 9L of ethyl acetate to the filtrate to divide Three times of extraction, distillation under reduced pressure at 45°C to obtain yellow oil N-ethyl-N-methylcarbamate-3-acetylphenyl ester (abbreviated as KB1), the yield is 85%, and the purity by HPLC is 98.7%
Embodiment 2
[0044] Synthesis of (R)-N-ethyl-N-methylcarbamic acid-3-(1-hydroxyethyl)phenyl ester
[0045] Put it in a microwave reactor under the protection of argon, take 0.25mol of diphenylcoaminoalcohol, dissolve it in anhydrous ethanol solution, add 0.42mol of trimethyl borate dropwise under stirring, control the dropping time at about 20min, and the dropping is completed Continue to react for 1h, add 2.0eq of borane tetrahydrofuran complex dropwise, dropwise for 20-30min, continue to stir for 30min, then slowly add KB1 3.72mol. dissolved in 7.5L of absolute ethanol, the reaction is exothermic, and dropwise The time is 80min, the microwave frequency is set to 80MHz, the synthesis reaction is continued for 16-18h, the reaction is stopped, the solvent is distilled off under reduced pressure, and the light yellow oily substance is obtained; 5L of ethyl acetate is added to the oily substance, and 4L of 1.5mol / L hydrochloric acid is added , stirred for 30min, static layering, retaining the...
Embodiment 3
[0047] Synthesis of free substance of rivastigmine
[0048] Under the protection of argon, place the material in an isotope energy level transition reactor, dissolve 3.8mol of KB2 in tetrahydrofuran solvent, cool down to 0-5°C, add 4.5mol of methanesulfonyl chloride dropwise, add dropwise for 60-80min, and replenish Add 4L of tetrahydrofuran solution, raise the temperature to 20°C, stir for 30 minutes, start the isotope energy level transition, control the temperature at 20°C-25°C and continue stirring for 5 hours, add 4L of water to quench the reaction, distill the solvent under reduced pressure, add 5L of ethyl acetate and stir Extract, separate the water layer, keep the organic layer, cool the organic layer to 0-5°C, add 3L of 2mol / L hydrochloric acid, stir, separate the organic layer, keep the water layer, add 3L of ethyl acetate to the water layer to extract twice, cool down Slowly add 2mol / L sodium hydroxide solution to 0-5°C to adjust the pH to 9-10. Extract with ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com