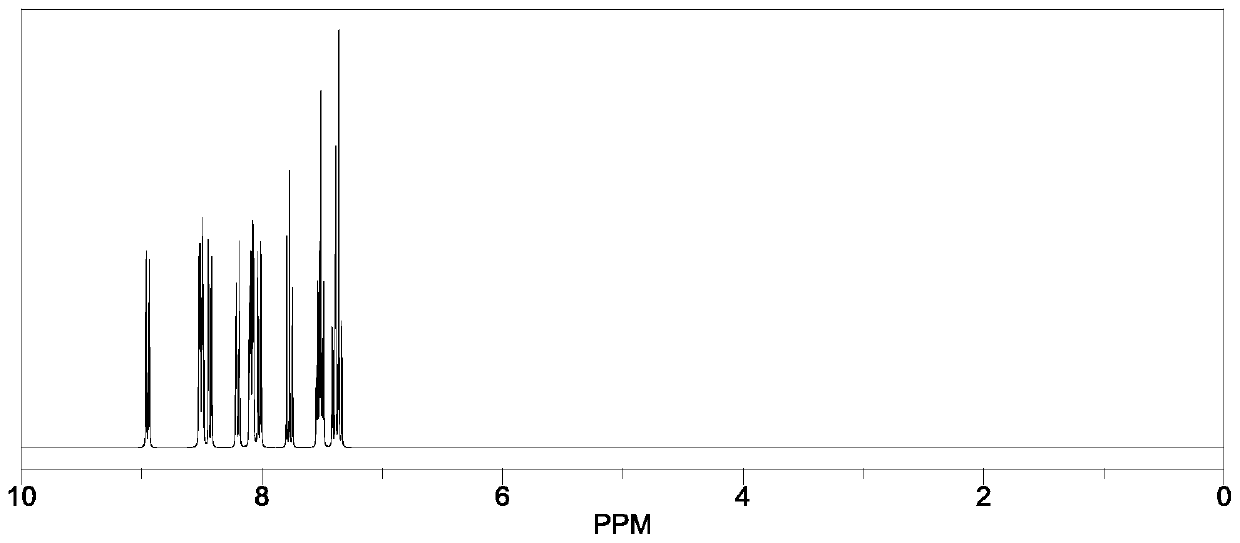
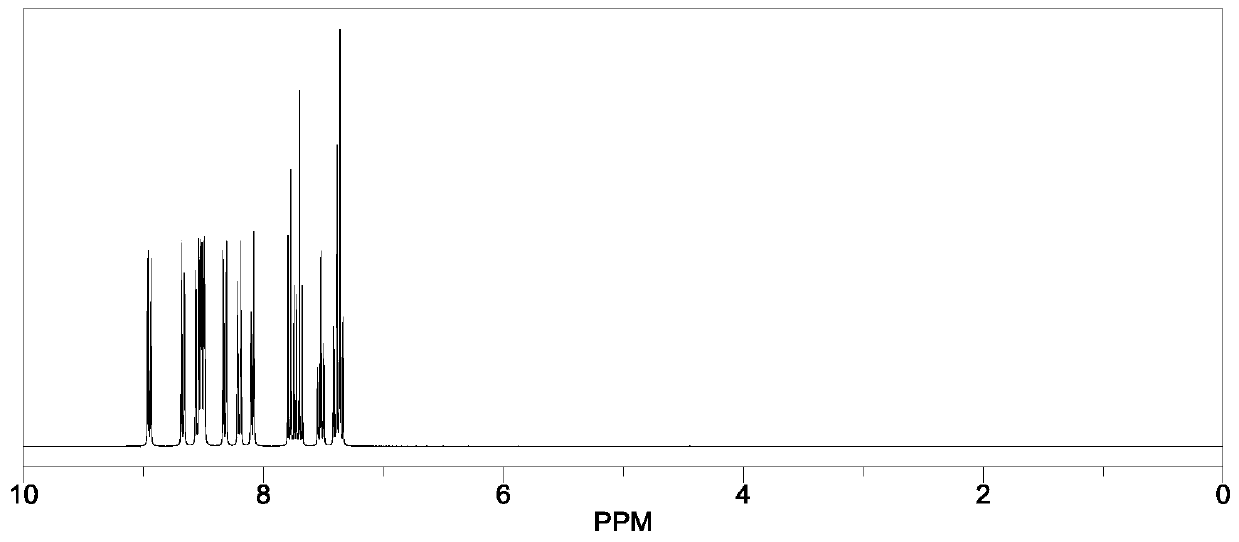
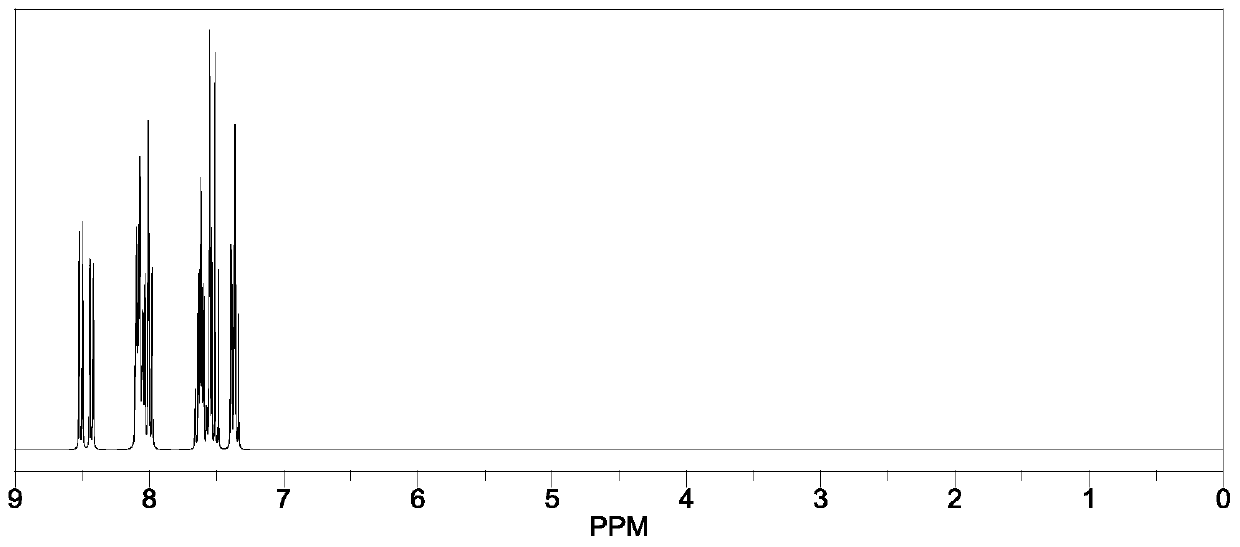
Heterocyclic compound, synthetic method thereof and organic light-emitting diode containing compound
A technology of heterocyclic compounds and synthesis methods, which is applied in the field of organic electroluminescent elements, can solve problems affecting the application of OLED devices, accelerate device attenuation, and reduce device efficiency, so as to increase charge transfer efficiency, prevent crystallization, and operate at low voltage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] Synthetic method of the present invention comprises the steps:
[0051] (1) Dissolve raw material Ia and raw material Ib in toluene / ethanol mixed solution (V ethanol: V toluene=20%-40%), add a certain amount of potassium carbonate, tetrabutylammonium bromide, tetratriphenyl Phosphine palladium, the reaction temperature is 50-100°C, the reaction time is 1-10 hours, follow the reaction of the raw materials, add 5-6 times (ml / mmol) of the theoretical amount of raw material Ia toluene for extraction, wash with water until neutral, and dry it in Distill under reduced pressure (pressure -0.08~-0.06MPa) at 30°C~60°C to concentrate to a small amount of solvent and recrystallize to obtain the first step product;
[0052]
[0053] (2) Dissolving the intermediate I in dichloroethane, adding a certain amount of cesium carbonate, the reaction temperature is 40°C-90°C, and the reaction time is 2-8 hours. The reaction solution was extracted with dichloroethane, washed with water u...
Embodiment 1
[0066] Embodiment 1: Compound 1 and its synthetic method
[0067] The structure of compound 1 is as follows:
[0068]
[0069] The synthetic method of above-mentioned compound 1, comprises the steps:
[0070] (1) Dissolve 10mmol of raw material 1a and 10mmol of raw material 1b in 50ml of toluene / ethanol mixed solution (V ethanol:V toluene=20%), under stirring, add 20mmol of potassium carbonate, 0.5mmol of tetrabutylammonium bromide, 0.02mmol of tetra Triphenylphosphine palladium, the reaction temperature is 50 DEG C, and the reaction time is 1 hour. After the reaction of the raw materials is completed, 50 ml of toluene is added for extraction, 100 ml of water is washed to neutrality, and after 10 g of anhydrous sodium sulfate is dried for 30 min, it is distilled under reduced pressure at 45 DEG C ( Pressure -0.06~-0.08MPa) concentrated to a small amount of solvent remaining and then recrystallized to obtain intermediate 11, the reaction equation is as follows:
[0071] ...
Embodiment 2
[0079] Embodiment 2: Compound 2 and its synthetic method
[0080] The structure of compound 2 is as follows:
[0081]
[0082] The synthetic method of above-mentioned compound 2, comprises the steps:
[0083] (1) Dissolve 10mmol of raw material 2a and 10mmol of raw material 2b in 70ml of toluene / ethanol mixed solution (V ethanol:V toluene=30%), under stirring, add 20mmol of potassium carbonate, 1mmol of tetrabutylammonium bromide, 0.02mmol of tetrathree Phenylphosphine palladium, the reaction temperature is 80°C, the reaction time is 3 hours, follow the reaction of the raw materials, add 50ml of toluene to extract, wash with 100ml of water until neutral, dry 30min with 10g of anhydrous sodium sulfate, and distill under reduced pressure at 45°C (pressure -0.06~-0.08MPa) concentrated to the remaining small amount of solvent and recrystallized to obtain intermediate 21, the reaction equation is as follows:
[0084]
[0085] (2) Dissolve 10 mmol of intermediate 21 in 50 ml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com