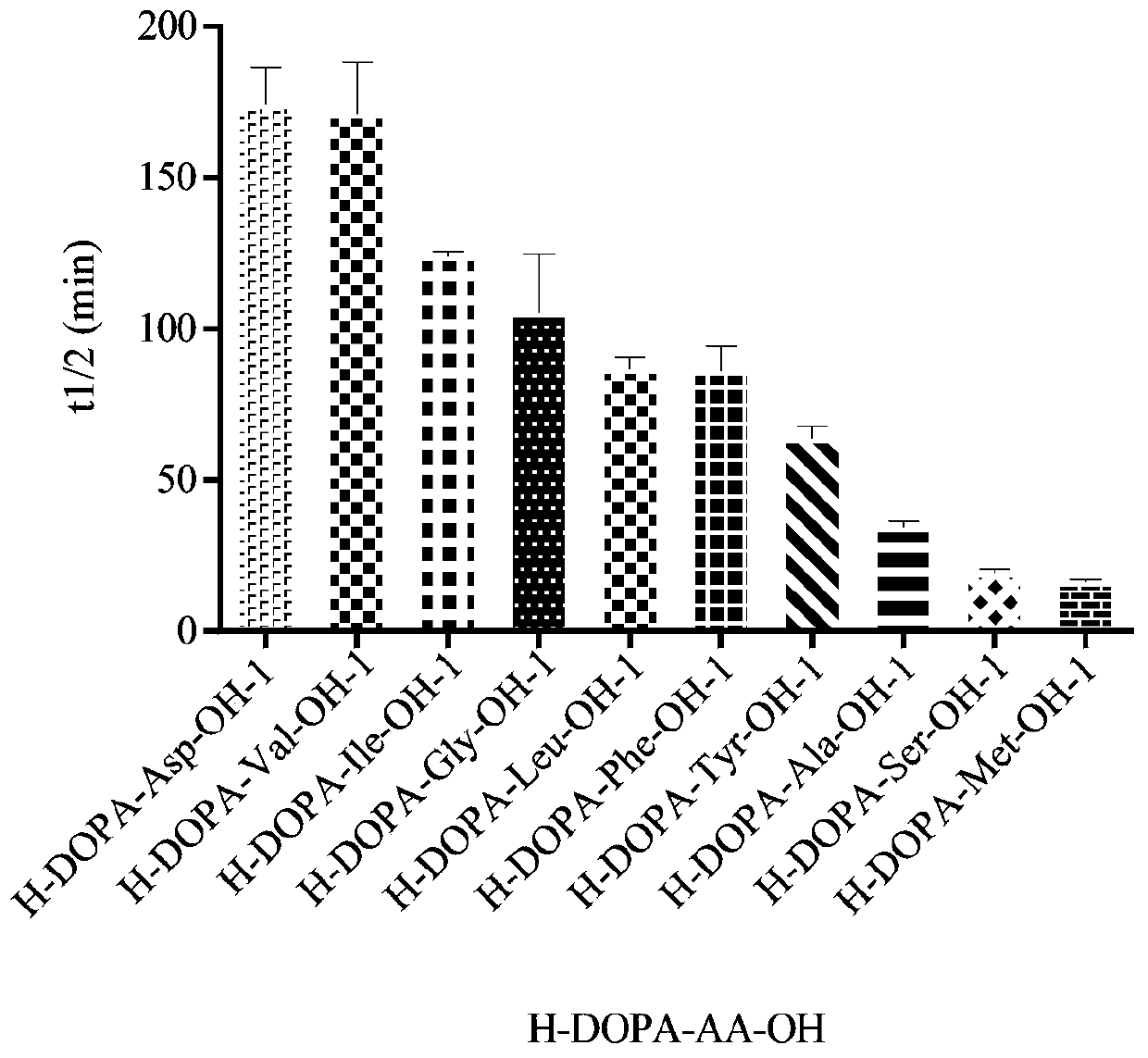
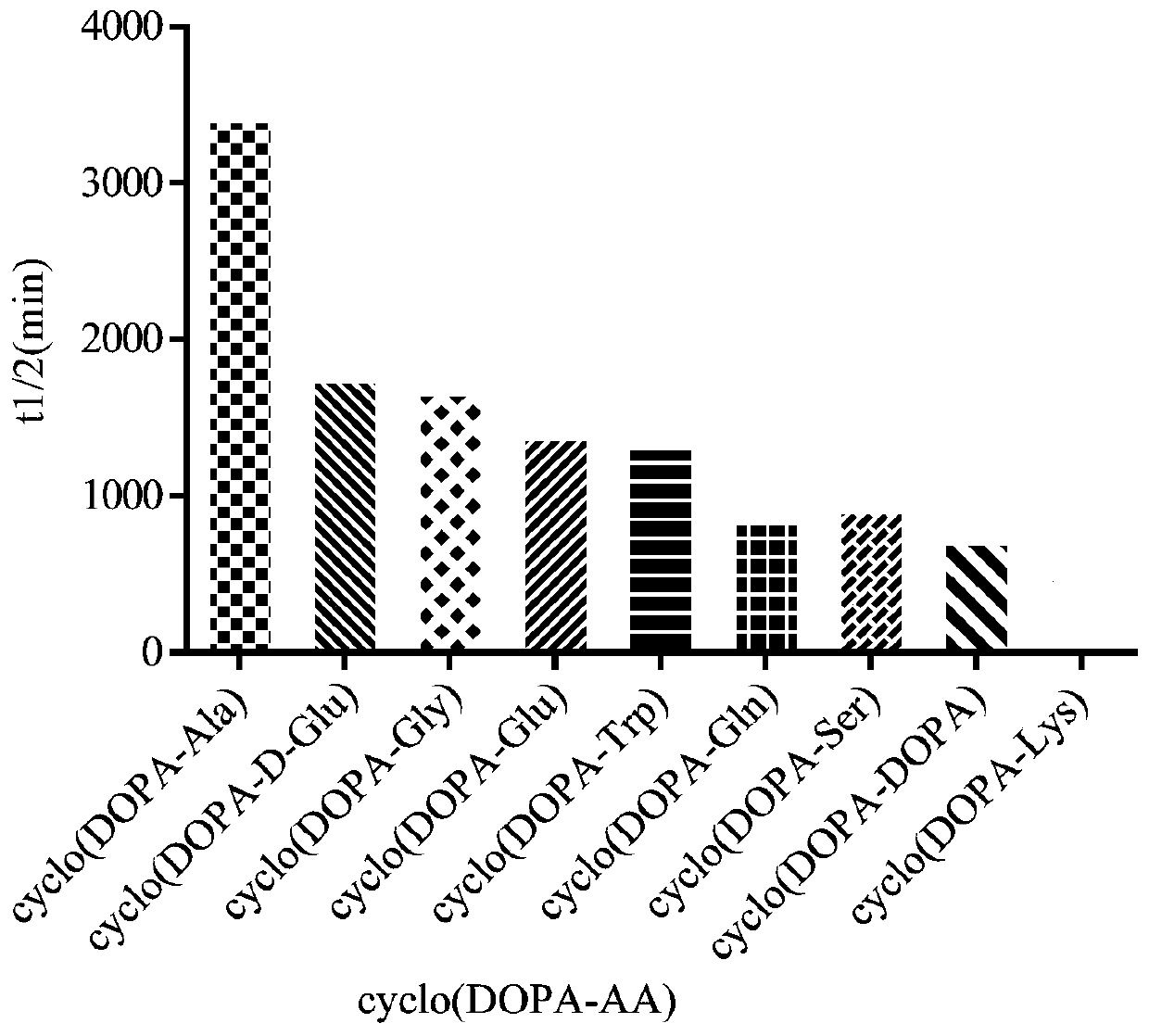
Synthesis method for dopa-containing oligopeptide and application of dopa-containing oligopeptide in antiparkinsonian prodrugs
A liquid-phase synthesis and synthetic route technology, which is applied in the preparation methods of peptides, medical preparations containing active ingredients, dipeptide ingredients, etc., can solve the problems of no inhibitory ability data, difficult to purchase, expensive and other problems, and achieves Good universality, improved bioavailability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
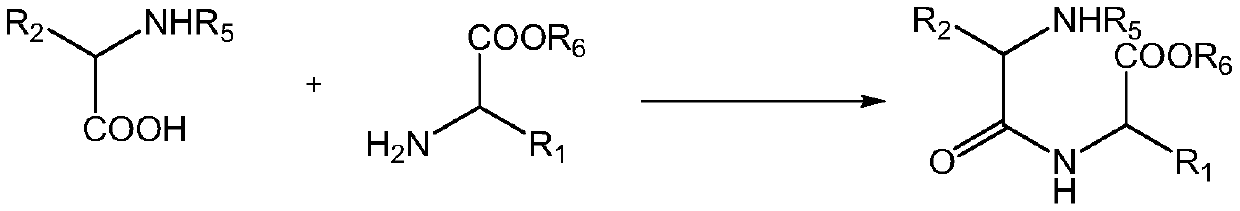
[0040] R 5 is an amino protecting group, R 6 For carboxyl protecting group; Synthetic method comprises the following steps:
[0041] Will Dissolve in organic solvent A, add condensing agent, liquid phase condensation reaction to obtain linear dipeptide R 5 -AA 1 -AA 2 -OR 6 ;
[0042] linear dipeptide R 5 -AA 1 -AA 2 -OR 6 Deprotection to obtain a linear dipeptide; or the linear dipeptide R 5 -AA 1 -AA 2 -OR 6 Dissolved in an alkaline deprotection solution for reaction, the liquid phase reaction removes the amino protecting group, and the protected dipeptide containing dopa ring is obtained
[0043] Amino acid side chains can be protected or not protected by general methods according to their reactivity. There is no special requirement for the type of amino acid, and it can be selected according to its specific use. Considering the wide range of raw material sources, easy availability and product safety, the amino acid side chain is preferably L-type or D-ty...
Embodiment 1
[0071] Embodiment 1, the synthesis of Cyclo (DOPA-Ala) (with the cyclic dipeptide that contains simple side chain amino acid formation)
[0072]
[0073] Dissolve Fmoc-DOPA(Acetonide)-OH (2.5mmol, 1.15g) and H-Ala-OMe HCl (2.5mmol, 0.35g) and HBTU (2.5mmol, 0.95g) in DMF (20ml), add DIEA (5mmol, 0.83ml), the solution was stirred at room temperature for 24 hours, then the solvent was evaporated in vacuo to a small amount. The resulting residue was added to saturated NaHCO 3 aqueous solution and ethyl acetate (1:1), with saturated NaHCO 3 Wash with aqueous solution 5 times. Then, the organic phase was washed with anhydrous Na 2 SO 4 dried, filtered to remove Na 2 SO 4 , the solvent was removed in vacuo, and recrystallized from a small amount of ethyl acetate and n-hexane to obtain the linear dipeptide Fmoc-DOPA(Acetonide)-Ala-OMe (1.07g, 79%). Fmoc-DOPA(Acetonide)-Ala-OMe(M:C 31 h 32 N 2 o 7 )([M+H] + :Calc.544.2210Found 544.2230).
[0074] The linear dipeptide F...
Embodiment 2
[0076] Embodiment 2, the synthesis of Cyclo (DOPA-Ser) (with the cyclic dipeptide that contains polar side chain amino acid formation)
[0077]
[0078] (1) Synthesis of compound Fmoc-DOPA(Acetonide)-Ser(tBu)-OMe
[0079] Dissolve Fmoc-DOPA(Acetonide)-OH (2.5mmol, 1.15g) and H-Ser(tBu)-OMe HCl (2.5mmol, 0.45g) and HBTU (2.5mmol, 0.95g) in DMF (100ml) , DIEA (5 mmol, 0.83 ml) was added and the solution was stirred at room temperature for 24 hours, then the solvent was evaporated in vacuo to a small amount. The resulting residue was added to saturated NaHCO 3 aqueous solution and ethyl acetate (1:1), with saturated NaHCO 3 Wash with aqueous solution 5 times. Then, the organic phase was washed with anhydrous Na 2 SO 4 dried, filtered to remove Na 2 SO 4 , the solvent was removed in vacuo, washed with a small amount of ethyl acetate, and filtered to give the linear dipeptide Fmoc-DOPA(Acetonide)-Ser(tBu)-OMe (1.03 g, 67%). Fmoc-DOPA(Acetonide)-Ser(tBu)-OMe(M:C 35 h 40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com